1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Kalali
Jul 13, 2025 · 7 min read
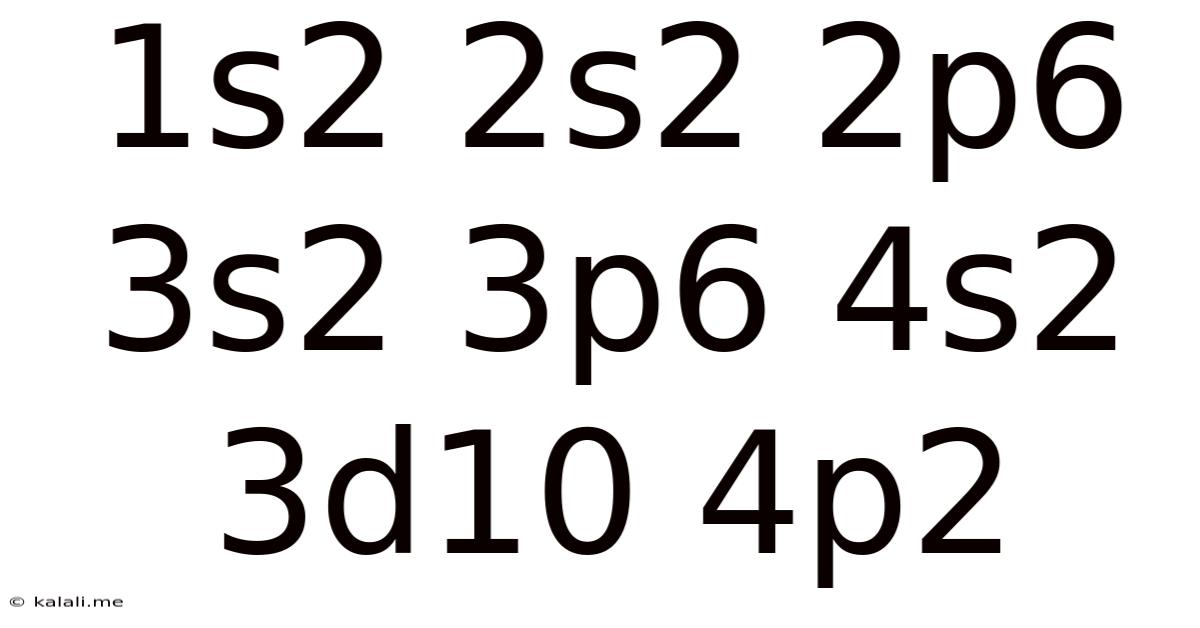
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²: Unveiling the Secrets of Lead's Electronic Structure
This seemingly cryptic string, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p², represents the electronic configuration of lead (Pb), a fascinating heavy metal with a rich history and diverse applications. Understanding this configuration unlocks a deeper appreciation for lead's unique chemical and physical properties. This article will delve into the intricacies of this electron arrangement, exploring its implications for lead's reactivity, bonding characteristics, and overall behavior. We will also touch upon the broader significance of electronic configurations in predicting the properties of elements across the periodic table.
What is an Electronic Configuration?
Before diving into the specifics of lead's configuration, let's establish a foundational understanding of what electronic configurations represent. An electronic configuration is a symbolic notation that describes the arrangement of electrons in the various energy levels (shells) and sublevels (subshells) within an atom. These energy levels are quantized, meaning electrons can only occupy specific energy states. The notation uses numbers and letters to indicate the principal quantum number (n), which represents the energy level, and the azimuthal quantum number (l), which defines the subshell (s, p, d, f). The superscript indicates the number of electrons in each subshell.
For instance, in the configuration 1s², the '1' signifies the first energy level (closest to the nucleus), 's' denotes the s subshell (which can hold a maximum of two electrons), and the '²' indicates that this subshell contains two electrons. This structured arrangement dictates an atom's reactivity, its ability to form chemical bonds, and its overall physical properties.
Deconstructing Lead's Electronic Configuration (1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p²)
Now, let's dissect the electronic configuration of lead: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p². This notation reveals the following:
-
Inner Shells (Core Electrons): The first three shells (1s² 2s² 2p⁶ 3s² 3p⁶) represent the core electrons. These electrons are tightly bound to the nucleus and are generally not involved in chemical bonding. They effectively shield the outer electrons from the full positive charge of the nucleus. This shielding effect influences the reactivity of the valence electrons.
-
Valence Electrons: The outermost electrons, found in the 4s and 4p subshells (4s² 4p²), are the valence electrons. These electrons participate directly in chemical bonding and determine lead's chemical properties. The presence of four valence electrons explains lead's ability to exhibit variable oxidation states, most commonly +2 and +4. This means lead can lose two or four electrons to form ions.
-
d-Orbital Filling: The 3d¹⁰ subshell is fully filled. The presence of a completely filled d subshell contributes to lead's relatively low reactivity compared to some transition metals. Filled d orbitals are quite stable and less prone to participation in chemical reactions.
Implications of Lead's Electronic Configuration
The electronic configuration has profound implications for lead's properties and behavior:
-
Chemical Reactivity: Lead's relatively low reactivity stems from its filled d subshell and the effective shielding provided by the core electrons. While it can participate in chemical reactions, it is less reactive than alkali metals or alkaline earth metals, which have only one or two valence electrons. The presence of four valence electrons allows for multiple oxidation states, contributing to the diversity of lead compounds.
-
Oxidation States: Lead displays two common oxidation states: +2 and +4. The +2 oxidation state is more stable and more frequently observed. This variability in oxidation states is a direct consequence of the four valence electrons. In the +2 state, lead loses the two 4p electrons, while in the +4 state, it loses both the 4p electrons and the two 4s electrons.
-
Bonding Characteristics: Lead readily forms ionic and covalent bonds. In ionic bonding, lead loses electrons to form cations (positively charged ions), such as Pb²⁺ and Pb⁴⁺. In covalent bonding, lead shares electrons with other atoms to achieve a stable electron configuration. The nature of the bonding dictates the properties of lead compounds.
-
Metallic Properties: Lead is a post-transition metal exhibiting typical metallic properties like good electrical conductivity (although lower than many other metals due to its relatively high atomic weight and complex electronic structure), malleability, and ductility. These properties are associated with the delocalized nature of the valence electrons in the metallic lattice.
-
Coordination Chemistry: The electronic configuration influences lead's coordination chemistry, describing how it interacts with ligands (molecules or ions that bind to the metal ion). The size of the lead ion, and its charge, dictate the number and type of ligands that can coordinate to it.
Lead's Applications and its Environmental Impact
Lead's unique properties have led to its extensive use in various applications throughout history, including:
-
Lead-acid batteries: The most significant application of lead is in lead-acid batteries, which are widely used in automobiles and other applications requiring reliable energy storage. The electrochemical reactions in these batteries leverage lead's ability to exhibit multiple oxidation states.
-
Radiation shielding: Lead's high atomic number makes it an effective shield against ionizing radiation, leading to its use in medical and industrial applications where radiation protection is crucial.
-
Pigments and paints: Lead-based pigments were once extensively used in paints, although their use has been drastically reduced due to their toxicity.
-
Soldering and alloys: Lead is added to various alloys to enhance their properties, such as increasing their melting point or improving their strength.
However, it is crucial to acknowledge the significant environmental and health concerns associated with lead. Lead is a toxic heavy metal, and exposure to lead can have severe neurological and developmental effects, particularly in children. Its widespread use in the past has resulted in significant environmental contamination, requiring extensive remediation efforts.
Comparative Analysis with Other Elements
Comparing lead's electronic configuration with those of other elements provides valuable insights into periodic trends. For example, comparing it to tin (Sn), another group 14 element, reveals similarities in valence electron configurations (Sn: 5s² 5p²) leading to similar bonding behavior in some compounds. However, differences in the inner shell electron configuration and the influence of relativistic effects account for some variations in properties between the two.
Furthermore, contrasting lead's configuration with those of transition metals highlights the differences in reactivity and bonding characteristics. Transition metals possess incomplete d orbitals, making them more reactive and prone to forming complex ions. Lead’s filled d-orbital contributes to its comparatively lower reactivity.
Relativistic Effects on Lead
For heavier elements like lead, relativistic effects play a crucial role in influencing their properties. These effects arise from the high velocity of the inner electrons, causing their mass to increase according to Einstein's theory of relativity. These relativistic effects influence the size of the atom, the energy levels of the electrons, and consequently, the chemical behavior of lead. The relativistic contraction of the 6s orbital, for example, results in a higher ionization energy, contributing to its lower reactivity.
Conclusion
The seemingly simple electronic configuration, 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p², encapsulates a wealth of information about lead's chemical and physical properties. Understanding this configuration allows us to comprehend its reactivity, bonding characteristics, oxidation states, and its consequent diverse applications. However, we must also acknowledge the environmental and health implications associated with lead and the necessity for responsible management and remediation efforts where lead contamination is present. Further research into lead's unique properties, especially those influenced by relativistic effects, continues to reveal insights into the fascinating world of heavy metal chemistry. The detailed examination of lead's electronic configuration thus serves as a gateway to understanding the fundamental principles that govern the behavior of elements in the periodic table and their interactions in the world around us.
Latest Posts
Latest Posts
-
What Happened To Christopher F Lima And Tim Loock
Jul 13, 2025
-
Gifts That Begin With The Letter M
Jul 13, 2025
-
What Fraction Is Equivalent To 1 2
Jul 13, 2025
-
What Is The Average Iq For A 9 Year Old
Jul 13, 2025
-
How Long Does It Take For Brandy Melville To Ship
Jul 13, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.