1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6
Kalali
Jul 21, 2025 · 6 min read
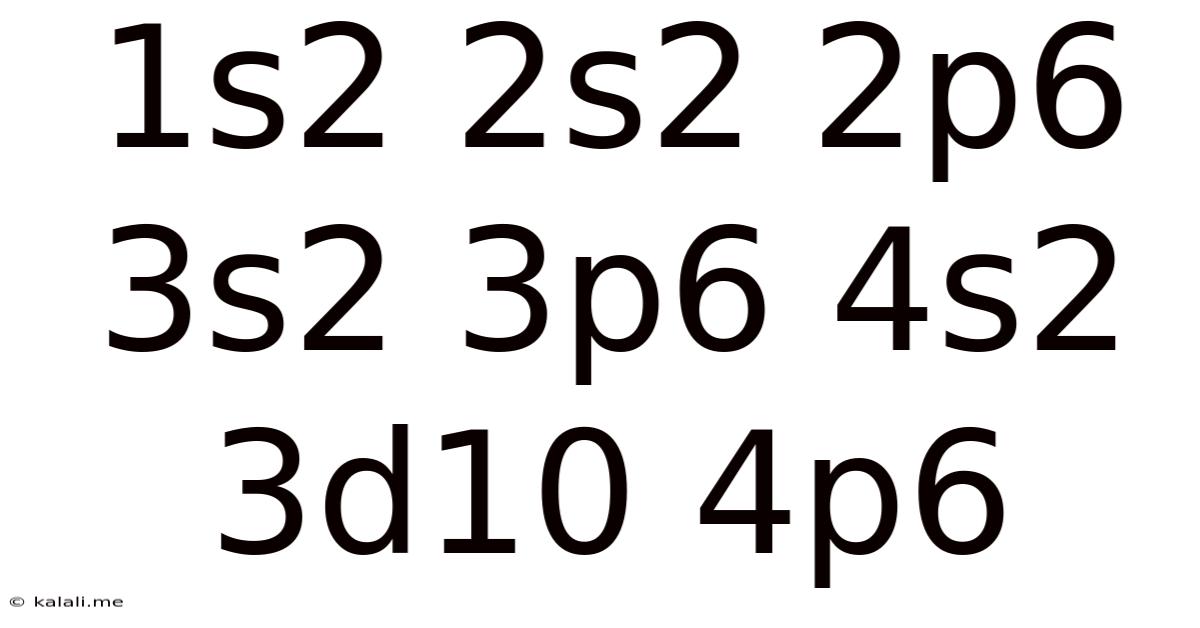
Table of Contents
Decoding 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶: A Deep Dive into Electron Configuration and its Implications
This seemingly cryptic string, "1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶", represents the electron configuration of a krypton atom (Kr). Understanding this notation unlocks a wealth of information about the atom's properties, behavior, and place within the periodic table. This article will delve into the meaning of this configuration, exploring the underlying principles of electron arrangement, its relationship to the periodic table, and its implications for chemical reactivity and physical properties.
Meta Description: Unravel the mysteries of the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶. Learn about electron shells, subshells, orbitals, and how this configuration dictates the properties of krypton.
Understanding Electron Configuration: The Building Blocks
Electron configuration describes how electrons are distributed among various energy levels within an atom. It follows the Aufbau principle, which states that electrons fill the lowest energy levels first. This arrangement is crucial because it determines an atom's chemical behavior and its position within the periodic table.
The notation uses numbers and letters to represent these energy levels:
-
Numbers (1, 2, 3, etc.): These represent the principal quantum number (n), indicating the energy level or shell. Higher numbers mean higher energy and greater distance from the nucleus.
-
Letters (s, p, d, f): These represent the azimuthal quantum number (l), specifying the subshell within a shell. Each subshell has a specific shape and can hold a certain number of electrons:
- s subshell: Spherical shape, holds up to 2 electrons.
- p subshell: Dumbbell shape, holds up to 6 electrons.
- d subshell: More complex shape, holds up to 10 electrons.
- f subshell: Even more complex shape, holds up to 14 electrons.
-
Superscripts (², ⁶, ¹⁰, etc.): These indicate the number of electrons occupying that particular subshell.
Deconstructing 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶
Let's break down the electron configuration of krypton (Kr) step-by-step:
-
1s²: The first energy level (n=1) contains one subshell, the s subshell, with two electrons.
-
2s²: The second energy level (n=2) has an s subshell with two electrons.
-
2p⁶: The second energy level also has a p subshell, which is filled with its maximum capacity of six electrons.
-
3s²: The third energy level (n=3) starts with an s subshell containing two electrons.
-
3p⁶: The third energy level also has a p subshell filled with six electrons.
-
4s²: The fourth energy level (n=4) begins with an s subshell holding two electrons. Notice that the 4s subshell fills before the 3d subshell, due to subtle energy level differences.
-
3d¹⁰: The third energy level finally has its d subshell filled with ten electrons.
-
4p⁶: The fourth energy level completes its p subshell with six electrons.
The Significance of a Filled Valence Shell
The outermost energy level, which contains the valence electrons, is crucial for determining an atom's chemical reactivity. In krypton's case, the valence shell (n=4) is completely filled with eight electrons (two in 4s and six in 4p). This full valence shell makes krypton an exceptionally stable and unreactive element, a characteristic of noble gases. They rarely form chemical bonds because they already possess a stable electron configuration.
Krypton's Position in the Periodic Table and its Properties
Krypton's electron configuration perfectly explains its position in the periodic table. It's a noble gas, belonging to Group 18, characterized by their inertness. Its filled valence shell is the reason for its lack of reactivity.
Some key properties of krypton, directly linked to its electron configuration, include:
-
Inertness: Krypton's reluctance to form chemical compounds.
-
Gas at Room Temperature: The weak interatomic forces between krypton atoms, a consequence of their filled valence shells, result in its gaseous state at standard conditions.
-
Low Boiling and Melting Points: Similar to other noble gases, krypton has very low boiling and melting points because of the weak intermolecular forces.
-
Colorless, Odorless, and Tasteless: Typical characteristics of noble gases due to their lack of interaction with other atoms.
Exceptions to the Aufbau Principle: A Closer Look
While the Aufbau principle generally holds true, there are exceptions, particularly involving the d and f subshells. These exceptions arise from subtle energy level interactions and electron-electron repulsions. For instance, some transition metals show deviations from the expected electron configuration due to the relatively close energy levels of the (n-1)d and ns orbitals. These exceptions highlight the complexities of electron interactions within an atom.
The filling order isn't always strictly sequential; sometimes a slightly higher energy level might be filled before a lower one to achieve a more stable configuration. This is often observed in transition metals and inner transition metals. Understanding these exceptions requires a deeper dive into quantum mechanics and atomic structure.
Applications of Krypton and its Isotopes
Despite its inertness, krypton finds applications in various fields, leveraging its unique properties:
-
Lighting: Krypton is used in fluorescent lights and high-intensity discharge lamps to enhance brightness and color.
-
Lasers: Krypton-based lasers are used in various applications, including surgery and spectroscopy.
-
Excimer Lasers: Krypton fluoride (KrF) excimer lasers produce ultraviolet light used in microlithography for semiconductor manufacturing.
-
Dating Techniques: Radioactive isotopes of krypton are utilized in radiometric dating techniques to determine the age of geological samples.
Beyond the Basics: Exploring Quantum Mechanics
A truly comprehensive understanding of electron configuration requires delving into the realm of quantum mechanics. Quantum numbers provide a more precise description of the electrons' state, including their energy, angular momentum, and spin.
The concepts of orbitals, wave functions, and probability densities are crucial for visualizing and interpreting the electron distribution within an atom. While the simple electron configuration notation gives a good overview, a deeper understanding of the underlying quantum mechanical principles is essential for accurately predicting and explaining atomic behavior.
Conclusion: The Power of Electron Configuration
The electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d¹⁰ 4p⁶ is more than just a string of numbers and letters; it's a concise summary of the fundamental properties of a krypton atom. It provides a framework for understanding its chemical behavior, its position in the periodic table, and its various applications. This seemingly simple notation unlocks a deeper understanding of the complexities of atomic structure and the principles governing chemical interactions, bridging the gap between seemingly abstract quantum mechanics and the tangible properties of matter. By mastering the interpretation of electron configurations, one gains a powerful tool for understanding the behavior of elements and their compounds. The implications extend far beyond a single element, providing the foundation for understanding chemical bonding, reactivity, and the diverse properties of the elements around us.
Latest Posts
Latest Posts
-
How Long After Expiration Date Is Miracle Whip Good
Jul 21, 2025
-
How Many Feet Are In 8000 Meters
Jul 21, 2025
-
Check Cashing Fees At Ace Cash Express
Jul 21, 2025
-
How Much Was A Cup Of Coffee In 1962
Jul 21, 2025
-
Believe Nothing You Hear And Half Of What You See
Jul 21, 2025
Related Post
Thank you for visiting our website which covers about 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.