5 Examples Of Chemical Potential Energy
Kalali
Mar 18, 2025 · 6 min read
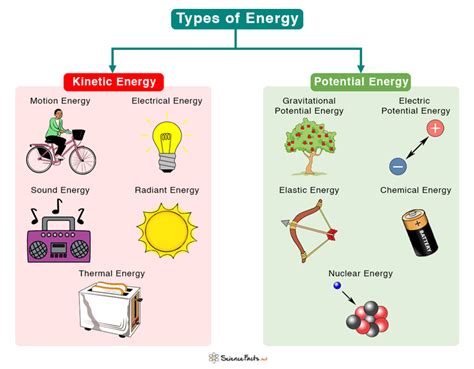
Table of Contents
5 Examples of Chemical Potential Energy: Unleashing the Power Within
Chemical potential energy is a form of stored energy that arises from the relative positions of atoms and molecules within a substance. It's the energy released or absorbed during a chemical reaction, a transformation driven by the rearrangement of these particles into more or less stable configurations. Understanding chemical potential energy is crucial to comprehending a wide range of phenomena, from the combustion of fuels to the complex processes within living organisms. This article will delve into five compelling examples of chemical potential energy, illustrating its significance in everyday life and beyond.
1. The Power of Fossil Fuels: Combustion and Energy Release
Fossil fuels – coal, oil, and natural gas – represent a concentrated form of chemical potential energy stored over millions of years. These fuels are essentially composed of complex hydrocarbon molecules, remnants of ancient plant and animal life. The high energy density of these molecules is a direct consequence of the strong chemical bonds between carbon and hydrogen atoms.
Understanding the Combustion Process
When fossil fuels are burned, a combustion reaction occurs. This involves a rapid oxidation process, where the carbon and hydrogen atoms in the fuel molecules react with oxygen in the air. This reaction breaks the existing bonds within the fuel molecules and forms new, more stable bonds in the products: carbon dioxide and water.
The key here is the difference in bond energies. The energy stored in the bonds of the reactants (fuel and oxygen) is significantly higher than the energy stored in the bonds of the products (carbon dioxide and water). This difference in energy is released as heat and light, providing the energy that powers vehicles, generates electricity in power plants, and heats our homes.
Environmental Considerations
While fossil fuels provide a readily available source of energy, their combustion contributes significantly to greenhouse gas emissions, primarily carbon dioxide. This has profound implications for climate change and necessitates exploring alternative, cleaner energy sources.
2. Batteries: Portable Chemical Energy Storage
Batteries are remarkable devices that convert chemical potential energy into electrical energy. They achieve this through redox reactions, which involve the transfer of electrons between different chemical species.
The Anode and Cathode Dance
A typical battery consists of two electrodes: an anode and a cathode, separated by an electrolyte. The chemical potential energy is stored in the materials of these electrodes. During discharge, a reduction reaction occurs at the cathode (gain of electrons), while an oxidation reaction occurs at the anode (loss of electrons). This electron flow constitutes the electrical current.
Different battery chemistries utilize different electrode materials, leading to variations in energy density, voltage, and lifespan. Lithium-ion batteries, for instance, are prevalent due to their high energy density and relatively long cycle life.
Rechargeable vs. Non-Rechargeable
Rechargeable batteries allow for the reversal of the chemical reactions through the application of an external electrical current, restoring the original chemical potential energy. In contrast, non-rechargeable batteries undergo irreversible chemical changes, rendering them unusable once discharged.
3. Food: Fueling Life Through Chemical Reactions
The food we consume is another excellent example of chemical potential energy. The complex molecules in our food – carbohydrates, proteins, and fats – contain significant amounts of stored energy within their chemical bonds.
Digestion and Energy Release
When we digest food, our bodies break down these complex molecules into simpler ones through a series of enzymatic reactions. These reactions release the chemical potential energy stored in the food molecules, providing the energy necessary for various bodily functions, such as movement, growth, and maintaining body temperature.
Cellular Respiration: The Energy Conversion Process
The primary process by which our bodies extract energy from food is cellular respiration. This process involves a series of carefully controlled oxidation-reduction reactions within our cells, ultimately converting the chemical potential energy in food into a usable form of energy – adenosine triphosphate (ATP). ATP then fuels a vast array of cellular processes.
Nutritional Value and Energy Content
Different foods contain varying amounts of chemical potential energy, reflected in their caloric content. Carbohydrates and fats generally provide more energy per gram than proteins. Understanding the nutritional value of different foods is crucial for maintaining a healthy diet and providing the body with the necessary energy for optimal function.
4. Explosives: Rapid Energy Release and Powerful Effects
Explosives represent a dramatic example of chemical potential energy, where a significant amount of energy is released rapidly and violently. These materials are carefully designed to undergo extremely exothermic reactions, producing a large volume of gas in a short period.
The Chemistry of Explosions
The chemical potential energy in explosives is stored in unstable molecules. When initiated (e.g., by impact, heat, or detonation), these molecules undergo a rapid decomposition reaction, breaking down into smaller, more stable molecules and releasing a large amount of energy in the form of heat, pressure, and light. This rapid expansion of gas produces the characteristic explosive effect.
Examples of Explosives
Common explosives include dynamite (nitroglycerin absorbed in diatomaceous earth), TNT (trinitrotoluene), and RDX (cyclotrimethylenetrinitramine). The specific chemical composition of an explosive determines its sensitivity, power, and detonation velocity.
Applications and Safety
Explosives have diverse applications, including mining, demolition, and military operations. However, their handling requires strict safety precautions due to the inherent dangers associated with their rapid energy release.
5. Photosynthesis: Capturing Solar Energy Through Chemical Reactions
Photosynthesis is a remarkable natural process where plants and other photosynthetic organisms convert light energy into chemical potential energy. This process is fundamental to life on Earth, providing the basis for most food chains.
Chlorophyll's Role
Photosynthesis takes place in chloroplasts, specialized organelles within plant cells containing chlorophyll. Chlorophyll absorbs light energy, initiating a series of reactions that convert carbon dioxide and water into glucose (a sugar) and oxygen.
Energy Storage in Glucose
Glucose is a relatively stable molecule, and its formation represents the storage of chemical potential energy derived from sunlight. This energy is then utilized by the plant for growth, reproduction, and other metabolic processes.
The Oxygen Cycle
The oxygen released during photosynthesis is a byproduct of the process and is essential for the respiration of many organisms, including humans. This process creates a vital cycle between photosynthesis and respiration, maintaining the balance of oxygen and carbon dioxide in the atmosphere.
Conclusion: The Ubiquity of Chemical Potential Energy
Chemical potential energy is a fundamental aspect of the physical world, influencing a vast range of phenomena from the energy that powers our civilization to the processes that sustain life on Earth. Understanding this form of energy provides valuable insights into the intricacies of chemical reactions and their profound impact on our lives and the environment. From the combustion of fossil fuels to the intricacies of photosynthesis, the power of chemical potential energy continues to shape our world in profound ways. Continued research and innovation in harnessing this energy responsibly will be crucial for a sustainable future.
Latest Posts
Latest Posts
-
11 Out Of 30 As A Percentage
Mar 19, 2025
-
Cuanto Es 92 Grados Fahrenheit En Centigrados
Mar 19, 2025
-
How To Get Magnitude Of Force
Mar 19, 2025
-
How Can You Separate Sugar From Water
Mar 19, 2025
-
What Are The Common Multiples Of 9 And 10
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about 5 Examples Of Chemical Potential Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
