A Diatomic Molecule With A Triple Covalent Bond Is
Kalali
Mar 31, 2025 · 5 min read
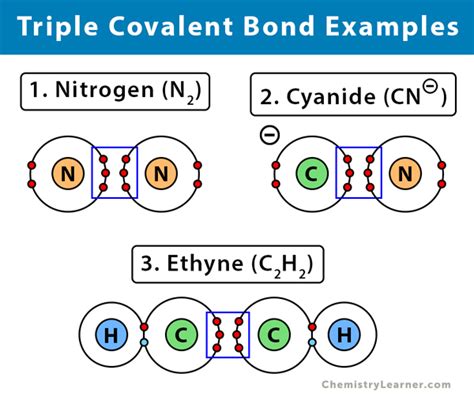
Table of Contents
A Diatomic Molecule with a Triple Covalent Bond Is: Exploring Nitrogen (N₂)
A diatomic molecule with a triple covalent bond is a fascinating example of chemical bonding. While several molecules fit this description, nitrogen gas (N₂) stands out due to its abundance and significance in the natural world. This article delves deep into the properties and characteristics of N₂, explaining its unique triple bond, exploring its implications, and touching upon its importance across various fields.
Understanding Covalent Bonds and Triple Bonds
Before we dive into the specifics of nitrogen, let's establish a foundational understanding of covalent bonds. A covalent bond is formed when two atoms share one or more pairs of electrons to achieve a more stable electron configuration, typically resembling a noble gas. This sharing occurs because the atoms involved have similar electronegativities; neither atom is strong enough to completely steal an electron from the other.
A single covalent bond involves the sharing of one electron pair. A double covalent bond involves the sharing of two electron pairs, and a triple covalent bond, as seen in N₂, involves the sharing of three electron pairs. This sharing leads to a stronger bond compared to single or double bonds, requiring significantly more energy to break.
The Nitrogen Molecule (N₂): A Deep Dive
Nitrogen, a ubiquitous element in the Earth's atmosphere (comprising roughly 78%), exists primarily as a diatomic molecule, N₂. This molecule is exceptionally stable due to the presence of its triple covalent bond. Let's unpack the significance of this:
The Triple Bond's Structure
Each nitrogen atom possesses five valence electrons. To achieve a stable octet (eight electrons in its outermost shell), each nitrogen atom shares three electrons with the other, forming three covalent bonds. This creates a strong, stable bond represented as N≡N. The triple bond consists of:
- One sigma (σ) bond: This is a strong, direct overlap of atomic orbitals, providing the foundational structure of the bond.
- Two pi (π) bonds: These are weaker bonds formed by the sideways overlap of p-orbitals. These bonds add to the overall strength and stability of the triple bond but are less robust than the sigma bond.
Implications of the Triple Bond
The triple bond in N₂ has several significant implications:
- High Bond Energy: Breaking the triple bond in N₂ requires a substantial amount of energy, resulting in a high bond dissociation energy. This high energy is responsible for nitrogen's remarkable inertness at room temperature. The strong bond makes it less reactive than many other diatomic molecules.
- Inertness and Stability: The strength and stability of the triple bond contribute significantly to nitrogen's relative inertness under normal conditions. This means it doesn't readily react with many other substances, a key factor in its atmospheric abundance.
- Unique Physical Properties: The triple bond influences the physical properties of nitrogen gas, such as its low boiling point (-196°C) and low density. This inertness makes it suitable for various applications, including cryogenic processes.
- Biological Significance: Despite its inertness, nitrogen is crucial for life. Nitrogen fixation, a process carried out by specific bacteria, converts atmospheric nitrogen (N₂) into usable forms like ammonia (NH₃) and nitrates (NO₃⁻). This conversion is essential for plant growth and the broader nitrogen cycle.
Nitrogen's Role in Various Fields
Nitrogen's unique properties and the characteristics of its triple bond have made it a versatile element used across many different fields:
Industrial Applications
- Fertilizers: The conversion of atmospheric nitrogen into ammonia through the Haber-Bosch process is a cornerstone of modern agriculture. Ammonia serves as a key ingredient in the production of nitrogenous fertilizers, supporting global food production.
- Refrigeration: Liquid nitrogen's extremely low boiling point makes it an ideal refrigerant for cryogenic applications, often used in the preservation of biological samples and materials.
- Welding and Metallurgy: Nitrogen is utilized in welding and metallurgical processes to create an inert atmosphere, preventing oxidation and contamination of molten metals.
- Chemical Synthesis: Nitrogen serves as a reactant in various chemical syntheses, including the production of nitric acid, which is used in numerous industrial processes.
- Packaging: Nitrogen gas is used as a protective atmosphere in food packaging to extend shelf life and prevent oxidation.
Medical Applications
- Cryosurgery: Liquid nitrogen's extreme cold is used in cryosurgery to freeze and destroy abnormal tissues, like cancerous cells.
- Medical Equipment Sterilization: Nitrogen gas can be used to create an inert atmosphere for sterilizing medical equipment, preventing microbial contamination.
Environmental Applications
- Wastewater Treatment: Nitrogen-based compounds are involved in wastewater treatment processes to remove harmful pollutants.
- Atmospheric Studies: Understanding nitrogen's role in the atmosphere, including its contribution to greenhouse gas emissions and its role in the formation of smog, is crucial for environmental management.
Contrasting with Other Triple-Bonded Diatomic Molecules
While nitrogen is the most prevalent example of a diatomic molecule with a triple bond, other elements also form such bonds, although often under different conditions. These include:
- Carbon Monoxide (CO): CO has a triple bond (C≡O), but its electronegativity difference makes it considerably more reactive than N₂. Its toxicity highlights its reactivity and its propensity to bind to hemoglobin, preventing oxygen transport in the blood.
- Cyanogen (C₂N₂): This molecule contains a carbon-carbon triple bond and features a linear structure. Its reactivity is higher compared to N₂.
The comparison highlights that while the presence of a triple bond is a common feature, the specific properties and reactivity of the molecule significantly depend on the atoms involved and their electron configurations.
Conclusion: The Significance of N₂'s Triple Bond
The triple covalent bond in the nitrogen molecule (N₂) is a striking example of chemical bonding's impact on a molecule's properties and behavior. Its strength and stability lead to nitrogen's inertness, influencing its abundance in the atmosphere and shaping its wide-ranging applications across numerous industries and scientific fields. Understanding the unique characteristics of this bond is crucial for appreciating the central role nitrogen plays in the natural world and human technological advancements. From the production of fertilizers supporting global food security to cryogenic applications in medicine and industry, the consequences of this remarkable bond are far-reaching and continuously evolving. The exploration of diatomic molecules with triple covalent bonds, like N₂, continues to be a vital area of research, driving innovation and deepening our understanding of the fundamental principles governing chemical interactions. The inherent stability and reactivity profiles of such molecules remain a captivating subject for ongoing scientific inquiry, promising further discoveries and advancements across numerous disciplines.
Latest Posts
Latest Posts
-
What Is The Percent Of 7 12
Apr 02, 2025
-
30 To The Power Of 2
Apr 02, 2025
-
What Is 400 Fahrenheit In Centigrade
Apr 02, 2025
-
50 Oz Is How Many Liters
Apr 02, 2025
-
How Many Feet Is 109 Inches
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about A Diatomic Molecule With A Triple Covalent Bond Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
