A Positive Ion Forms When An Atom ______.
Kalali
Mar 29, 2025 · 5 min read
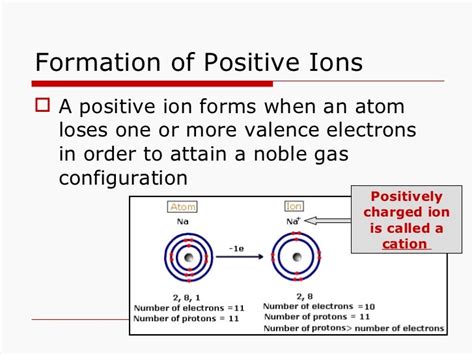
Table of Contents
A Positive Ion Forms When an Atom Loses an Electron
A fundamental concept in chemistry and physics is the formation of ions. Understanding how ions form is crucial to grasping various chemical reactions, the behavior of matter, and the properties of materials. This article delves into the process of positive ion formation, explaining the underlying principles and providing examples to solidify your understanding.
The Basics: Atoms and Ions
Before diving into positive ion formation, let's establish a solid foundation. An atom is the basic unit of matter, comprising a nucleus (containing protons and neutrons) and orbiting electrons. Protons carry a positive charge, electrons carry a negative charge, and neutrons are neutral. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
An ion, on the other hand, is an atom or molecule that carries a net electrical charge. This charge arises from an imbalance in the number of protons and electrons. When an atom gains electrons, it becomes a negative ion (anion). Conversely, when an atom loses electrons, it becomes a positive ion (cation). The process of ion formation is known as ionization.
A Positive Ion Forms When an Atom Loses an Electron: The Mechanism
The core statement, "A positive ion forms when an atom loses an electron," encapsulates the fundamental principle. This electron loss occurs due to several reasons:
1. Electrostatic Forces and Electronegativity:
Electronegativity is a crucial factor determining whether an atom will lose or gain electrons. Electronegativity represents an atom's ability to attract electrons towards itself in a chemical bond. Atoms with low electronegativity tend to lose electrons readily, while those with high electronegativity tend to gain electrons. When an atom with low electronegativity interacts with an atom possessing significantly higher electronegativity, the atom with lower electronegativity is likely to lose one or more electrons to the more electronegative atom. This is driven by the electrostatic forces—the positive nucleus of the highly electronegative atom attracts the loosely held electron of the less electronegative atom.
2. Ionization Energy:
Ionization energy is the minimum energy required to remove an electron from a neutral gaseous atom. The lower the ionization energy of an atom, the easier it is to remove an electron and form a positive ion. Elements with low ionization energies, typically found on the left side of the periodic table (alkali metals and alkaline earth metals), readily lose electrons to achieve a stable electron configuration.
3. Achieving a Stable Electron Configuration (Octet Rule):
Atoms strive to attain a stable electron configuration, often resembling the electron configuration of a noble gas. Noble gases have a full outermost electron shell (except for helium, which has a full shell with two electrons), making them chemically inert. Many atoms achieve stability by losing electrons to attain this noble gas configuration, resulting in a positive ion. For instance, sodium (Na) has one electron in its outermost shell. By losing this electron, it achieves the stable electron configuration of neon (Ne), forming a Na⁺ ion.
Examples of Positive Ion Formation:
Let's illustrate positive ion formation with specific examples:
1. Sodium (Na) forming Na⁺:
Sodium, an alkali metal, has an electronic configuration of 2, 8, 1. It readily loses its single valence electron to achieve the stable electron configuration of neon (2, 8). This loss of an electron results in a sodium cation (Na⁺), with a +1 charge.
Na → Na⁺ + e⁻
Where 'e⁻' represents the lost electron.
2. Magnesium (Mg) forming Mg²⁺:
Magnesium, an alkaline earth metal, has an electronic configuration of 2, 8, 2. It loses two valence electrons to achieve the stable configuration of neon (2, 8), resulting in a magnesium cation (Mg²⁺) with a +2 charge.
Mg → Mg²⁺ + 2e⁻
3. Aluminum (Al) forming Al³⁺:
Aluminum has an electronic configuration of 2, 8, 3. It loses three valence electrons to achieve the stable neon configuration (2, 8), forming an aluminum cation (Al³⁺) with a +3 charge.
Al → Al³⁺ + 3e⁻
4. Transition Metals:
Transition metals exhibit more complex ionization behavior. They can lose varying numbers of electrons from their outermost and penultimate shells, leading to cations with different charges. For example, iron (Fe) can form Fe²⁺ and Fe³⁺ ions.
Factors Influencing Positive Ion Formation:
Several factors influence the ease and extent of positive ion formation:
- Atomic Size: Larger atoms generally have lower ionization energies and tend to lose electrons more readily.
- Nuclear Charge: A higher nuclear charge (more protons) leads to a stronger attraction for electrons, making it more difficult to remove them.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. Greater shielding reduces the effective nuclear charge experienced by outer electrons, making them easier to remove.
- Electron Configuration: Atoms with loosely held valence electrons, especially those with only one or two valence electrons, are more prone to lose electrons.
Applications of Positive Ions:
Positive ions play a significant role in various applications:
- Electrochemistry: Positive ions are essential components in electrochemical cells (batteries), conducting electricity through solutions.
- Materials Science: The properties of many materials, such as ceramics and semiconductors, are strongly influenced by the presence of positive ions.
- Biological Systems: Positive ions, such as sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺), are crucial for numerous biological processes, including nerve impulse transmission and muscle contraction.
- Mass Spectrometry: Mass spectrometry relies on ionizing atoms or molecules to measure their mass-to-charge ratio, allowing for the identification and quantification of different species.
- Plasma Technology: Plasmas, which are ionized gases containing a significant number of positive and negative ions, are used in various technologies, including lighting, etching, and surface modification.
Conclusion:
The formation of a positive ion is a fundamental process driven by the electrostatic attraction between the nucleus and electrons, the atom's desire to achieve a stable electron configuration, and its ionization energy. Understanding this process is key to grasping a wide range of chemical and physical phenomena, from simple chemical reactions to complex biological processes and advanced technological applications. The examples provided illustrate the diverse ways in which positive ions form and their essential role in various aspects of our world. Further exploration into the intricacies of ionization energy, electronegativity, and electron configurations will enrich your understanding of this crucial concept in chemistry.
Latest Posts
Latest Posts
-
What Is A 35 Out Of 50
Mar 31, 2025
-
How Many Grams Are In 25 Mg
Mar 31, 2025
-
How Many Lines Of Symmetry Does A Cross Have
Mar 31, 2025
-
How Many 12 Glasses In Quart
Mar 31, 2025
-
69 Inches Is How Many Feet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about A Positive Ion Forms When An Atom ______. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
