A Raw Egg Is Fried Physical Or Chemical Change
Kalali
Mar 12, 2025 · 6 min read
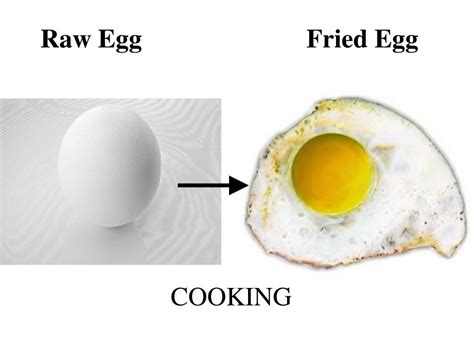
Table of Contents
Is Frying a Raw Egg a Physical or Chemical Change? A Deep Dive
Frying an egg is a common kitchen task, but have you ever stopped to consider the underlying science? Is the transformation from a runny, translucent egg white to a firm, opaque mass a physical change or a chemical change? The answer, as we'll explore in detail, is both. This seemingly simple process involves a complex interplay of physical and chemical transformations, making it a fascinating example of matter changing states and properties.
Understanding Physical and Chemical Changes
Before delving into the specifics of frying an egg, let's establish a clear understanding of the core concepts:
Physical Changes
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of cutting paper, melting ice, or dissolving sugar in water. These processes change the physical state or shape of the substance, but the fundamental molecules remain unchanged. The substance can often be returned to its original state through a simple physical process (e.g., freezing water back into ice).
Chemical Changes
A chemical change, also known as a chemical reaction, results in the formation of new substances with different chemical properties. These changes are often irreversible and involve the breaking and forming of chemical bonds. Examples include burning wood, rusting iron, or cooking an egg. The original substances are transformed into entirely new compounds with distinct characteristics.
The Physical Changes in Frying an Egg
When you fry an egg, several physical changes occur:
1. Temperature-Induced State Changes:
- Heat Transfer: The primary physical change is the transfer of heat from the pan to the egg. This energy causes an increase in the egg's temperature.
- Water Evaporation: Egg white and yolk contain a significant amount of water. As the temperature rises, this water begins to evaporate, turning from a liquid to a gas. This contributes to the egg's thickening and the formation of a firm surface. The loss of water is purely a physical change, as the water molecules remain water molecules even in their gaseous form.
- Protein Denaturation (Initial Stage): Although protein denaturation is fundamentally a chemical change, the initial stages involve a physical unfolding of the protein molecules. Before the chemical bonds break, the proteins begin to unravel and change their shape, which is a physical alteration. This is observable in the thickening and setting of the egg white.
2. Changes in Texture and Viscosity:
The heat causes the egg white and yolk to transition from a liquid to a semi-solid, then eventually a solid-like state. This alteration in texture and viscosity is a physical change, as the basic components of the egg (proteins, fats, water) remain largely the same. The change in consistency is a result of the interactions between these components at different temperatures. You could, in theory, reverse the process by adding enough water to reconstitute some of the original state but would not achieve the same original egg quality.
The Chemical Changes in Frying an Egg
While the physical changes are significant, the frying process is predominantly driven by chemical changes, primarily concerning the proteins within the egg:
1. Protein Denaturation:
This is the most crucial chemical change. Egg proteins, primarily albumin in the white and various proteins in the yolk, exist in a complex, folded structure in their raw state. When heated, the weak bonds (hydrogen bonds) that maintain this folded structure break. This causes the protein molecules to unfold and become entangled with each other. This process, known as denaturation, is irreversible, resulting in a hardened, solidified structure. The chemical composition of the protein hasn't changed significantly—the same amino acids are present—but their arrangement and bonding patterns have drastically altered.
2. Maillard Reaction:
The Maillard reaction is a chemical reaction between amino acids and reducing sugars that occurs when food is heated. This reaction is responsible for the characteristic browning and development of flavors and aromas in cooked food. In the case of a fried egg, the Maillard reaction contributes to the color changes observed in the egg white and yolk as they cook, particularly at higher temperatures and longer cooking times. The reaction creates hundreds of new flavor compounds, impacting the taste and smell of the fried egg. This is an unmistakable chemical change, creating entirely new molecules.
3. Lipid Oxidation:
The egg yolk contains fats (lipids). Prolonged exposure to high temperatures can lead to lipid oxidation, a chemical process where fats react with oxygen. This can result in the formation of undesirable flavors and off-odors, contributing to the rancid taste sometimes experienced in overcooked eggs. Lipid oxidation is a complex chemical process resulting in the breakdown and formation of new molecules, altering the chemical composition of the egg's fat content.
Differentiating Physical and Chemical Changes in the Frying Process
It's important to emphasize that the line between physical and chemical changes in frying an egg is blurry. Protein denaturation, for example, begins with a physical unfolding of the protein, but ultimately involves the formation of new chemical bonds between the unfolded protein molecules. Evaporation is a purely physical change, yet it significantly impacts the overall physical properties of the egg.
The Maillard reaction and lipid oxidation are clear examples of chemical changes that create new molecules, altering the color, flavor, aroma, and nutritional value of the egg. These reactions don't occur to any significant degree at low temperatures, making the precise point of transition between a mostly physical process and a predominantly chemical process difficult to define.
The degree of each type of change also depends on factors like cooking temperature, cooking time, and the presence of other ingredients (e.g., oil, seasonings). A quickly fried egg will undergo less extensive chemical reactions than a slowly cooked, over-easy egg.
Conclusion: A Synergistic Transformation
Frying a raw egg is a compelling demonstration of how physical and chemical changes work together. The initial stages involve significant physical changes—temperature increases, water evaporation, and protein unfolding. However, these physical transitions pave the way for irreversible chemical changes, primarily protein denaturation, the Maillard reaction, and lipid oxidation. These chemical reactions alter the egg's chemical composition, creating the cooked egg's unique texture, flavor, color, and aroma. Understanding this interplay of physical and chemical processes allows us to better appreciate the complexities of even the simplest culinary procedures.
Further Exploration:
To deepen your understanding of this topic, you can explore:
- The chemistry of proteins: Learn more about the structure and properties of proteins, and how they behave under different conditions.
- The Maillard reaction: Research this fascinating chemical reaction in greater detail, focusing on the various compounds produced and their impact on food flavor and aroma.
- Lipid oxidation: Understand the mechanisms of lipid oxidation and its effects on food quality and nutrition.
By investigating these areas further, you can gain a more comprehensive appreciation of the scientific principles underlying everyday cooking processes. The humble fried egg, therefore, serves as a surprisingly complex and rewarding case study in the dynamics of matter transformations.
Latest Posts
Latest Posts
-
What Is 2 3 Of 3 Quarts
Jul 02, 2025
-
Courtesy Is Cumbersome To Them That Know It Not
Jul 02, 2025
-
How Far Is 220 Yards On A Track
Jul 02, 2025
-
What Is The Shortest Chapter In The Bible
Jul 02, 2025
-
What Has 4 Letters Sometimes Has 9
Jul 02, 2025
Related Post
Thank you for visiting our website which covers about A Raw Egg Is Fried Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.