A Substance That Combines With Hydrogen Ions
Kalali
Apr 07, 2025 · 6 min read
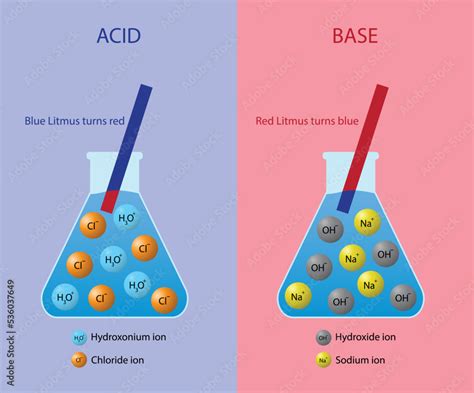
Table of Contents
A Substance That Combines with Hydrogen Ions: A Deep Dive into Bases and Their Reactions
Understanding chemical reactions is fundamental to comprehending the world around us. One crucial type of reaction involves substances that combine with hydrogen ions (H⁺), commonly known as bases. This article will explore the fascinating world of bases, delving into their definitions, properties, classifications, reactions, and their widespread applications across various industries.
What are Bases?
In chemistry, a base is a substance that can accept or receive hydrogen ions (protons) or donate hydroxide ions (OH⁻) when dissolved in water. This fundamental property defines their behavior in aqueous solutions and their ability to neutralize acids. The key characteristic differentiating a base from other substances lies in its interaction with acids. When a base reacts with an acid, a neutralization reaction occurs, forming water and a salt.
Arrhenius Definition of Bases
The simplest definition of a base comes from Svante Arrhenius. According to the Arrhenius theory, a base is a substance that dissociates in water to produce hydroxide ions (OH⁻). For example, sodium hydroxide (NaOH) dissociates completely in water to form sodium ions (Na⁺) and hydroxide ions (OH⁻):
NaOH(aq) → Na⁺(aq) + OH⁻(aq)
This increase in hydroxide ions raises the pH of the solution, making it alkaline.
Brønsted-Lowry Definition of Bases
A broader definition is provided by the Brønsted-Lowry theory. This theory defines a base as a proton acceptor. This definition extends beyond substances that produce hydroxide ions, encompassing molecules or ions that can accept a proton (H⁺) from an acid. For instance, ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water, forming ammonium ions (NH₄⁺) and hydroxide ions (OH⁻):
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
Lewis Definition of Bases
The most comprehensive definition comes from the Lewis theory. According to Gilbert N. Lewis, a base is an electron-pair donor. This definition encompasses a wider range of substances, including those that don't necessarily contain hydroxide ions or accept protons directly. A Lewis base donates a lone pair of electrons to form a coordinate covalent bond with a Lewis acid (an electron-pair acceptor). For example, ammonia (NH₃) acts as a Lewis base by donating its lone pair of electrons to a hydrogen ion (H⁺), forming an ammonium ion (NH₄⁺).
Properties of Bases
Bases exhibit several characteristic properties that distinguish them from acids:
- Taste: Bases typically have a bitter taste. (Note: Never taste chemicals in a laboratory setting.)
- Feel: Many bases feel slippery or soapy to the touch. This is due to their reaction with oils and fats on the skin.
- pH: Bases have a pH greater than 7. The pH scale measures the concentration of hydrogen ions in a solution; a higher pH indicates a lower concentration of H⁺ and a higher concentration of OH⁻.
- Reaction with Acids: Bases react with acids in a neutralization reaction, producing water and a salt.
- Electrical Conductivity: Aqueous solutions of strong bases are good conductors of electricity due to the presence of ions.
- Effect on Indicators: Bases change the color of certain indicators, such as litmus paper (turning it blue) and phenolphthalein (turning it pink).
Classification of Bases
Bases can be classified into several categories based on their properties and behavior:
Strong Bases vs. Weak Bases
Strong bases completely dissociate in water, producing a high concentration of hydroxide ions. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)₂).
Weak bases only partially dissociate in water, producing a lower concentration of hydroxide ions. Examples include ammonia (NH₃) and many organic amines.
Alkali Metals Hydroxides
The hydroxides of alkali metals (Group 1 elements) are strong bases. They readily dissolve in water to release hydroxide ions. These bases are highly reactive and corrosive.
Alkaline Earth Metals Hydroxides
The hydroxides of alkaline earth metals (Group 2 elements) are generally weaker bases compared to alkali metal hydroxides. Their solubility in water is lower.
Reactions of Bases
Bases participate in a variety of important chemical reactions:
Neutralization Reactions
The most characteristic reaction of a base is its neutralization reaction with an acid. This reaction involves the combination of hydrogen ions (H⁺) from the acid and hydroxide ions (OH⁻) from the base to form water (H₂O). A salt, an ionic compound formed from the cation of the base and the anion of the acid, is also produced.
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
Reactions with Metal Oxides
Bases react with certain metal oxides to form salts and water.
Reactions with Non-metal Oxides
Bases react with some non-metal oxides to form salts and water. This reaction is often used to remove acidic gases from industrial emissions.
Applications of Bases
Bases have a wide range of applications in various fields:
Industrial Applications
- Manufacturing: Bases are used extensively in the production of soaps, detergents, and other cleaning agents. They are also used in the manufacturing of paper, textiles, and other materials.
- Chemical Processing: Bases are important reactants and catalysts in many industrial chemical processes.
- Wastewater Treatment: Bases are used to neutralize acidic wastewater before it is discharged into the environment.
Everyday Applications
- Cleaning Products: Many household cleaning products, such as drain cleaners and oven cleaners, contain strong bases.
- Food Industry: Bases are used as food additives and in the processing of certain foods.
- Medicine: Some bases are used in medications to treat various conditions.
Other Applications
- Agriculture: Bases are used to adjust the pH of soil.
- Environmental Remediation: Bases are used to neutralize acidic spills and other environmental pollutants.
Safety Precautions
Many bases are corrosive and can cause severe burns to skin and eyes. Always wear appropriate personal protective equipment (PPE), such as gloves, goggles, and lab coats, when handling bases. In case of contact with skin or eyes, immediately flush the affected area with plenty of water and seek medical attention.
Conclusion
Bases are essential chemical substances with a broad range of properties and applications. Understanding their nature, reactions, and safety precautions is crucial for anyone working with chemicals or interested in chemistry. From industrial processes to everyday household uses, bases play a significant role in our lives. This article provides a comprehensive overview of these important compounds and their diverse functions, highlighting their significance in various scientific and practical contexts. Further research into specific bases and their applications can provide an even deeper understanding of this fascinating area of chemistry. The exploration of the different definitions and classifications of bases allows for a more nuanced perspective on their interactions within chemical systems. Furthermore, understanding the safety protocols associated with the handling of bases is paramount to ensuring the safety of oneself and the surrounding environment.
Latest Posts
Latest Posts
-
How Many Hours In 165 Minutes
Apr 09, 2025
-
111 Is What Percent Of 300
Apr 09, 2025
-
Cuanto Es 8 Pies En Pulgadas
Apr 09, 2025
-
How Many Inches In 6 Meters
Apr 09, 2025
-
How Many Meters In 10 Yards
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about A Substance That Combines With Hydrogen Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.