Are Metalloids Or Nonmetals Good Conductors Of Heat And Electricity
Kalali
Mar 12, 2025 · 6 min read
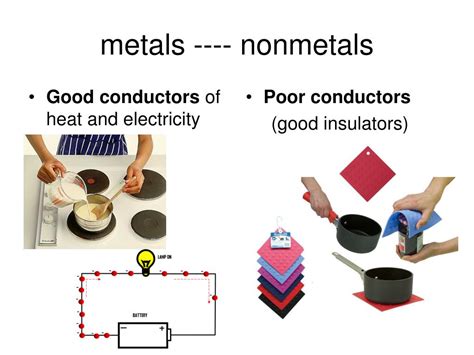
Table of Contents
Are Metalloids or Nonmetals Good Conductors of Heat and Electricity?
The ability of a material to conduct heat and electricity is a fundamental property determined by its atomic structure and the behavior of its electrons. While metals are renowned for their excellent conductivity, the conductivity of metalloids and nonmetals is significantly more nuanced and varies greatly depending on the specific element and its crystalline structure. This article delves deep into the electrical and thermal conductivity of metalloids and nonmetals, exploring the underlying reasons for their behavior and comparing them to the superior conductivity of metals.
Understanding Electrical Conductivity
Electrical conductivity describes a material's ability to allow the flow of electric current. This flow is facilitated by the movement of electrons. In metals, a "sea" of delocalized electrons is readily available to carry charge, leading to high conductivity. However, metalloids and nonmetals have different electronic structures that significantly impact their conductive capabilities.
Metals: The Champions of Conductivity
Metals possess a unique electronic structure. Their valence electrons are loosely bound to their atoms and are free to move throughout the metal lattice. This mobility of electrons allows for the easy passage of electric current when a voltage is applied. This is why metals like copper, silver, and aluminum are extensively used in electrical wiring.
Nonmetals: Poor Conductors, Mostly
Nonmetals, on the other hand, generally exhibit poor electrical conductivity. Their valence electrons are tightly bound to their atoms, making electron mobility extremely limited. Consequently, very few electrons are available to carry charge, resulting in high electrical resistance. Examples of poor conductors include sulfur, phosphorus, and oxygen. However, there are exceptions; some nonmetals can exhibit conductivity under specific conditions. For example, carbon in its graphite form is a relatively good conductor due to its unique layered structure and the delocalized electrons within those layers.
Metalloids: A Bridging Behavior
Metalloids represent a fascinating intermediate category between metals and nonmetals. Their electrical conductivity lies somewhere between these two extremes. This variability depends on several factors, including:
- Temperature: The conductivity of some metalloids increases with increasing temperature, unlike metals which show a decrease. This semi-conducting behavior is crucial in their applications in electronic devices.
- Doping: The intentional addition of impurities (doping) to metalloids can dramatically alter their conductivity. Doping with specific elements can create either n-type (electron-rich) or p-type (electron-deficient) semiconductors, forming the basis of many electronic components.
- Crystalline Structure: The arrangement of atoms in a metalloid's crystal lattice significantly impacts its conductivity. Different structural forms of the same metalloid can exhibit vastly different electrical properties.
Examples of Metalloid Conductivity:
Silicon and germanium are prime examples of metalloids exhibiting semi-conducting behavior. Their electrical conductivity is significantly lower than metals but higher than typical nonmetals. This semi-conducting nature makes them indispensable in the creation of transistors and integrated circuits. Other metalloids, like arsenic and boron, exhibit similar semi-conducting behavior but with varying degrees of conductivity.
Understanding Thermal Conductivity
Thermal conductivity refers to a material's ability to transfer heat energy. Similar to electrical conductivity, this property is intimately linked to the material's atomic structure and electron behavior.
Metals: Efficient Heat Transporters
Metals are excellent thermal conductors. The free-moving electrons responsible for their high electrical conductivity also play a crucial role in their heat transfer capabilities. When one part of a metal is heated, the kinetic energy of the electrons increases. These energized electrons rapidly transfer this energy throughout the metal lattice, resulting in efficient heat distribution. This is why metals like copper and aluminum are frequently used in cookware and heat sinks.
Nonmetals: Poor Heat Conductors
Nonmetals generally exhibit poor thermal conductivity. The tightly bound electrons limit their ability to transport thermal energy. Heat transfer in nonmetals primarily occurs through lattice vibrations (phonons). However, the efficiency of phonon-mediated heat transfer is significantly lower than electron-mediated transfer in metals. Therefore, nonmetals like wood, rubber, and plastics are frequently used as insulators.
Metalloids: A Middle Ground Again
Similar to their electrical conductivity, the thermal conductivity of metalloids falls between metals and nonmetals. Their thermal conductivity is higher than many nonmetals but significantly lower than most metals. The behavior is also affected by factors such as temperature and crystalline structure. Silicon, for instance, while a good semiconductor, is not as effective a heat conductor as metals like copper. This limited thermal conductivity necessitates the use of effective heat sinks in silicon-based electronic devices to prevent overheating.
Factors Affecting Conductivity in Metalloids and Nonmetals
Several key factors influence the conductivity of both metalloids and nonmetals:
-
Band Gap: The energy difference between the valence band (where electrons reside in their ground state) and the conduction band (where electrons can move freely) is called the band gap. In insulators and semiconductors (including metalloids), this band gap is relatively large. A larger band gap indicates greater difficulty for electrons to jump into the conduction band, thus leading to lower conductivity. In metals, the conduction and valence bands overlap, facilitating electron movement.
-
Temperature: Temperature affects conductivity differently in metals and semiconductors. In metals, increased temperature leads to increased vibrations of the atoms, hindering electron flow and thus reducing conductivity. In semiconductors, however, increased temperature provides more energy for electrons to overcome the band gap, leading to increased conductivity.
-
Impurities and Defects: The presence of impurities or defects in the crystal structure can significantly alter the conductivity of metalloids and nonmetals. Impurities can create additional energy levels within the band gap, affecting the number of charge carriers available for conduction. This is the basis for doping in semiconductor technology.
-
Pressure: Applying external pressure can influence the atomic spacing and electronic structure of metalloids and nonmetals, altering their conductivity.
Applications Leveraging Conductivity Properties
The unique conductivity properties of metalloids and nonmetals are exploited in a vast array of applications:
-
Semiconductors: Silicon and germanium, crucial metalloids, form the foundation of modern electronics. Their semi-conducting nature allows for the controlled flow of electricity, making them essential components in transistors, integrated circuits, and microprocessors.
-
Insulators: Nonmetals like rubber, plastics, and ceramics are excellent electrical and thermal insulators. They are widely used in electrical wiring, building materials, and protective coatings.
-
Thermoelectric Materials: Some metalloids and their compounds exhibit thermoelectric properties, meaning they can convert heat energy directly into electrical energy or vice versa. This is useful in applications such as power generation from waste heat.
-
Photovoltaic Cells: Certain metalloids and nonmetals are used in photovoltaic cells (solar cells) to convert sunlight into electricity. The unique electronic properties of these materials allow them to absorb photons and generate electron-hole pairs, leading to a current.
Conclusion: A Spectrum of Conductivity
In summary, the conductivity of metalloids and nonmetals is far from uniform. While metals excel as excellent conductors of both heat and electricity due to their freely moving electrons, metalloids occupy a unique space as semiconductors, exhibiting conductivity that can be significantly altered by various factors. Nonmetals, on the other hand, are typically poor conductors, relying on less efficient phonon-mediated heat transfer. Understanding these differences is crucial for designing and developing countless technological applications that leverage the unique properties of these materials. The interplay between band gap, temperature, impurities, and crystal structure profoundly impacts the conductivity of these materials, leading to their diverse range of applications across various industries. Further research into novel materials and advanced techniques continues to push the boundaries of conductivity manipulation, paving the way for new innovations in electronics, energy, and beyond.
Latest Posts
Latest Posts
-
If I Was Born In 1987 How Old Am I
Jul 05, 2025
-
Whats The Average Iq For A 13 Year Old
Jul 05, 2025
-
How Many Peters Are In The Bible
Jul 05, 2025
-
Which Situation Is An Example Of An Internal Conflict
Jul 05, 2025
-
What Year Was I Born If Im 65
Jul 05, 2025
Related Post
Thank you for visiting our website which covers about Are Metalloids Or Nonmetals Good Conductors Of Heat And Electricity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.