Barium Hydroxide Strong Or Weak Base
Kalali
Mar 27, 2025 · 6 min read
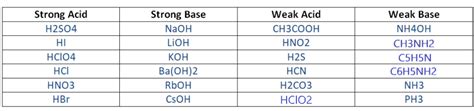
Table of Contents
Barium Hydroxide: A Deep Dive into its Strength as a Base
Barium hydroxide, with its chemical formula Ba(OH)₂, is a fascinating inorganic compound that sparks considerable interest in the realms of chemistry and its applications. A key point of discussion surrounding barium hydroxide centers on its classification as a strong base. This article will delve deep into the properties and characteristics of barium hydroxide, examining its behavior in solutions and providing evidence to support its classification as a strong base. We will also explore its various applications and safety considerations.
Understanding the Concept of Strong and Weak Bases
Before diving into the specifics of barium hydroxide, let's establish a clear understanding of what constitutes a strong base versus a weak base. A base, in simple terms, is a substance that accepts protons (H⁺ ions) or donates hydroxide ions (OH⁻ ions) in a solution. The strength of a base is determined by its ability to completely or partially dissociate in water.
-
Strong Bases: Strong bases completely dissociate into their constituent ions in aqueous solutions. This means that essentially all of the base molecules break apart into hydroxide ions (OH⁻) and their conjugate acid cation. This leads to a high concentration of hydroxide ions, resulting in a highly alkaline solution.
-
Weak Bases: Weak bases only partially dissociate in water. This implies that only a small fraction of the base molecules break down into hydroxide ions and their conjugate acid cation. The equilibrium lies heavily towards the undissociated base molecules, resulting in a lower concentration of hydroxide ions and a less alkaline solution.
The Evidence: Why Barium Hydroxide is a Strong Base
Several lines of evidence strongly support the classification of barium hydroxide as a strong base:
1. Complete Dissociation in Water
The most crucial piece of evidence lies in the behavior of barium hydroxide when dissolved in water. Barium hydroxide readily and completely dissociates into its constituent ions: barium ions (Ba²⁺) and hydroxide ions (OH⁻). The reaction can be represented as follows:
Ba(OH)₂(s) → Ba²⁺(aq) + 2OH⁻(aq)
This complete dissociation is a hallmark characteristic of strong bases. The absence of undissociated Ba(OH)₂ molecules in the solution is key to understanding its strength.
2. High pH Values
Strong bases produce highly alkaline solutions with high pH values. When barium hydroxide is dissolved in water, the resulting solution exhibits a very high pH, typically above 12. This high pH is a direct consequence of the high concentration of hydroxide ions produced by the complete dissociation of the base. The higher the pH, the stronger the base.
3. Conductivity Measurements
Electrolytic conductivity is another indicator of the strength of a base. Strong bases, due to their complete dissociation into ions, are excellent conductors of electricity. Solutions of barium hydroxide exhibit high electrical conductivity, further supporting its classification as a strong base. This is because the freely moving ions (Ba²⁺ and OH⁻) can easily carry an electric current.
4. Titration Experiments
Titration experiments, which involve the controlled neutralization of an acid with a base, provide quantitative evidence for the strength of a base. Titration curves for strong bases show a sharp equivalence point, indicative of complete neutralization. Barium hydroxide exhibits this characteristic sharp equivalence point in titration experiments, further validating its status as a strong base.
Applications of Barium Hydroxide
The strong basicity of barium hydroxide makes it useful in a variety of applications:
1. Industrial Applications
- Sugar Refining: Barium hydroxide is used in the refining of sugar cane and beet sugar. It helps in the precipitation of impurities, leading to a purer sugar product.
- Chemical Synthesis: It serves as a reagent in several chemical synthesis reactions, particularly in the production of other barium compounds.
- Water Treatment: In some niche applications, it can be used for water treatment, although its toxicity necessitates careful handling and stringent safety protocols. This use is less common due to safer and more readily available alternatives.
- Manufacturing of other compounds: Barium hydroxide plays a crucial role in producing various barium salts used in diverse industries. The high reactivity of its hydroxide ions facilitates efficient reactions.
2. Laboratory Applications
- Analytical Chemistry: It finds use in analytical chemistry as a standard base in titrations. Its high purity and complete dissociation make it suitable for precise quantitative analyses.
- Preparation of other bases: Barium hydroxide can be used to synthesize other less soluble metal hydroxides through precipitation reactions.
Safety Precautions: Handling Barium Hydroxide
It is crucial to emphasize the importance of safety when handling barium hydroxide. It is a toxic substance, and direct contact can cause irritation, burns, and more serious health consequences. Always follow these safety measures:
- Personal Protective Equipment (PPE): Wear appropriate PPE, including gloves, eye protection, and lab coats, when handling barium hydroxide.
- Ventilation: Work in a well-ventilated area to avoid inhaling dust or fumes.
- Disposal: Dispose of barium hydroxide waste according to local regulations. Do not pour it down the drain.
- First Aid: In case of contact with skin or eyes, immediately flush the affected area with plenty of water and seek medical attention. Ingestion requires immediate medical intervention.
Comparison with Other Bases
To further solidify the understanding of barium hydroxide's strength, let's compare it with other bases:
- Sodium Hydroxide (NaOH): Similar to barium hydroxide, sodium hydroxide (NaOH) is also a strong base that completely dissociates in water. However, barium hydroxide's higher molar mass leads to a greater amount of hydroxide ions released per gram of the substance.
- Ammonia (NH₃): In contrast to barium hydroxide, ammonia is a weak base. It only partially dissociates in water, resulting in a much lower concentration of hydroxide ions.
- Calcium Hydroxide [Ca(OH)₂]: While also a strong base, calcium hydroxide has a lower solubility in water compared to barium hydroxide. This means that although it completely dissociates the available dissolved molecules, it produces fewer hydroxide ions in a saturated solution than barium hydroxide.
The difference in strength is a result of the inherent properties of the hydroxide and the metal cation involved. The barium cation has a larger ionic radius and a lower charge density compared to smaller cations like sodium or calcium. This relatively lower electrostatic attraction towards the hydroxide ions results in a more readily dissociated hydroxide ion and thus, a stronger base.
Conclusion: Barium Hydroxide's Strong Base Nature
In summary, overwhelming evidence supports the classification of barium hydroxide as a strong base. Its complete dissociation in water, high pH values, high conductivity, titration behavior, and comparison with other bases all point to its strong base characteristics. However, it is essential to remember its toxicity and always handle it with appropriate safety precautions. Understanding the strength and properties of barium hydroxide is critical for its safe and effective use in various industrial and laboratory applications. This knowledge is vital for anyone working with this compound, ensuring both safety and effective utilization of its unique chemical properties.
Latest Posts
Latest Posts
-
What Is 30 Percent Of 800
Mar 30, 2025
-
How Many Liters Is 100 Ml
Mar 30, 2025
-
1 In 25 As A Percentage
Mar 30, 2025
-
For Each Action There Is A Reaction
Mar 30, 2025
-
Why Do Flowers Contain More Stamen Than Pistils
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Barium Hydroxide Strong Or Weak Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
