Calculate Ph At The Equivalence Point
Kalali
Mar 17, 2025 · 6 min read
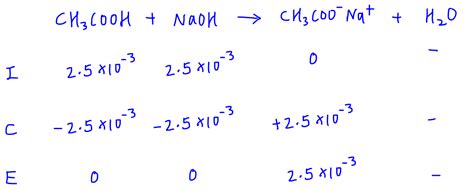
Table of Contents
Calculating pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial in analytical chemistry, providing valuable insights into the strength of acids and bases. This point signifies the complete neutralization of the analyte (the substance being analyzed) by the titrant (the solution of known concentration). Understanding how to calculate this pH is essential for accurate quantitative analysis. This comprehensive guide will walk you through the different scenarios, providing clear explanations and practical examples.
Understanding the Equivalence Point
The equivalence point isn't visually apparent during a titration; it's a theoretical point where the moles of acid and base are stoichiometrically equal. This is different from the endpoint, which is the point where the indicator changes color, signaling the approximate equivalence point. Ideally, the endpoint and equivalence point are very close, but a small difference can exist depending on the indicator used.
The pH at the equivalence point depends heavily on the nature of the acid and base involved:
-
Strong Acid-Strong Base Titration: The pH at the equivalence point is always 7. This is because the resulting salt formed is neutral. The complete neutralization leaves only water and a neutral salt.
-
Weak Acid-Strong Base Titration: The pH at the equivalence point will be greater than 7 (basic). This is because the conjugate base of the weak acid is a weak base itself, and undergoes hydrolysis, increasing the hydroxide ion concentration.
-
Strong Acid-Weak Base Titration: The pH at the equivalence point will be less than 7 (acidic). Similar to the previous case, the conjugate acid of the weak base is a weak acid, undergoing hydrolysis and increasing the hydronium ion concentration.
-
Weak Acid-Weak Base Titration: The pH at the equivalence point will be closer to 7, but it's difficult to predict exactly without considering the specific Ka and Kb values. The calculation requires considering the hydrolysis of both the conjugate acid and conjugate base.
Calculating pH at the Equivalence Point for Different Titration Types
1. Strong Acid-Strong Base Titration
This is the simplest case. Since both the acid and base are completely dissociated, the pH at the equivalence point is 7 (neutral). No further calculations are needed. For example, in the titration of HCl (strong acid) with NaOH (strong base), at the equivalence point, the solution contains only NaCl and water, resulting in a neutral pH.
2. Weak Acid-Strong Base Titration
This scenario requires a more involved calculation. Here's a step-by-step approach:
Step 1: Determine the moles of weak acid initially present.
This is calculated by multiplying the initial volume of the weak acid by its initial molar concentration.
Step 2: Determine the moles of strong base added at the equivalence point.
At the equivalence point, the moles of the strong base added are equal to the initial moles of the weak acid.
Step 3: Calculate the concentration of the conjugate base.
The moles of conjugate base formed are equal to the moles of the initial weak acid. Divide this by the total volume (initial volume of acid + volume of base added) to find the concentration of the conjugate base.
Step 4: Use the Kb expression to find the hydroxide ion concentration.
The Kb of the conjugate base can be calculated from the Ka of the weak acid using the relationship Kw = Ka * Kb, where Kw is the ion product constant of water (1.0 x 10⁻¹⁴ at 25°C).
The Kb expression for the conjugate base, A⁻, is:
Kb = [OH⁻][HA] / [A⁻]
Since [OH⁻] = [HA], we can simplify:
Kb = [OH⁻]² / [A⁻]
Solve for [OH⁻].
Step 5: Calculate the pOH and then the pH.
pOH = -log[OH⁻]
pH = 14 - pOH
Example:
Consider the titration of 25.0 mL of 0.100 M acetic acid (CH₃COOH, Ka = 1.8 x 10⁻⁵) with 0.100 M NaOH.
-
Moles of CH₃COOH: (0.025 L)(0.100 mol/L) = 0.0025 mol
-
Moles of NaOH at equivalence point: 0.0025 mol (equal to moles of CH₃COOH)
-
Volume of NaOH at equivalence point: 0.0025 mol / 0.100 mol/L = 0.025 L = 25.0 mL
-
Total volume: 25.0 mL + 25.0 mL = 50.0 mL = 0.050 L
-
Concentration of CH₃COO⁻: 0.0025 mol / 0.050 L = 0.050 M
-
Kb of CH₃COO⁻: Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰
-
[OH⁻]: Solving the Kb expression: [OH⁻] = √(Kb * [CH₃COO⁻]) = √(5.6 x 10⁻¹⁰ * 0.050) ≈ 5.3 x 10⁻⁶ M
-
pOH: pOH = -log(5.3 x 10⁻⁶) ≈ 5.28
-
pH: pH = 14 - 5.28 ≈ 8.72
3. Strong Acid-Weak Base Titration
The approach is very similar to the weak acid-strong base titration, but instead of using Kb, you'll use Ka for the conjugate acid of the weak base. The pH at the equivalence point will be less than 7. The calculations involve determining the concentration of the conjugate acid formed and using the Ka expression to find the hydronium ion concentration.
4. Weak Acid-Weak Base Titration
This is the most complex scenario. The pH at the equivalence point is dependent on both the Ka of the weak acid and the Kb of the weak base. A simplified approach involves calculating the concentration of the conjugate acid and conjugate base and then using an ICE table to determine the equilibrium concentrations and subsequently calculate the pH. However, often this requires iterative calculations or the use of more advanced techniques. This is beyond the scope of a basic introduction but demonstrates the complexity of this specific case.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point, including:
-
Temperature: The Kw of water varies with temperature, influencing the calculations involving weak acids and bases.
-
Ionic Strength: The presence of other ions in the solution can affect the activity of the ions involved in the equilibrium, slightly altering the pH.
-
Concentration of the Acid and Base: The concentration influences the precision of the calculations, with very dilute solutions leading to increased uncertainties.
Conclusion
Calculating the pH at the equivalence point is fundamental to understanding acid-base titrations. While the strong acid-strong base titration is straightforward, the calculations for weak acid/base titrations involve equilibrium considerations and require a deeper understanding of acid-base chemistry. Mastering these calculations provides a critical foundation for various analytical chemistry applications. Remember to always consider the specific nature of the acid and base involved to choose the appropriate calculation method and accurately determine the pH at the equivalence point. Further exploration into more advanced techniques and computational methods can enhance the accuracy and efficiency of these calculations in complex situations.
Latest Posts
Latest Posts
-
How Many Cm Is 108 Inches
Mar 17, 2025
-
Does Boiling Sea Water Make It Drinkable
Mar 17, 2025
-
Which Evolutionary Adaptations Helped Plants Succeed And Spread On Land
Mar 17, 2025
-
What Is 4 15 As A Percent
Mar 17, 2025
-
Is A Squirrel A Primary Consumer
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Calculate Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
