Calculating The Ph At The Equivalence Point
Kalali
Mar 15, 2025 · 6 min read
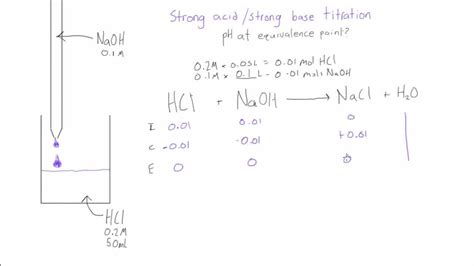
Table of Contents
Calculating the pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and selecting appropriate indicators. This point signifies the complete neutralization of the analyte, marking a significant change in the solution's properties. This comprehensive guide explores various scenarios, providing step-by-step calculations and explanations to master this essential concept in chemistry.
Understanding the Equivalence Point
The equivalence point in a titration is the point at which the moles of titrant added equal the moles of analyte present in the solution. This doesn't necessarily mean the pH is 7; it depends on the nature of the acid and base involved. For example:
- Strong Acid - Strong Base Titration: The equivalence point pH is 7. Complete neutralization results in a neutral salt solution.
- Weak Acid - Strong Base Titration: The equivalence point pH is greater than 7. The conjugate base of the weak acid hydrolyzes, producing hydroxide ions and increasing the pH.
- Strong Acid - Weak Base Titration: The equivalence point pH is less than 7. The conjugate acid of the weak base hydrolyzes, producing hydronium ions and decreasing the pH.
- Weak Acid - Weak Base Titration: The equivalence point pH depends on the relative strengths of the acid and base. Predicting the exact pH is more complex and requires considering the Ka and Kb values.
Calculating pH at the Equivalence Point: Different Scenarios
Let's delve into the calculations for each scenario, using practical examples and explanations.
1. Strong Acid - Strong Base Titration
This is the simplest case. The salt formed is neutral, resulting in a pH of 7 at 25°C. Consider the titration of 25.00 mL of 0.100 M HCl with 0.100 M NaOH.
Steps:
- Determine the moles of acid: Moles HCl = (0.100 mol/L) * (0.02500 L) = 0.00250 mol
- Determine the volume of base at equivalence: Since the concentrations are equal, the volume of NaOH required is equal to the volume of HCl: 25.00 mL.
- Calculate the total volume: Total volume = 25.00 mL + 25.00 mL = 50.00 mL = 0.05000 L
- Since it's a strong acid-strong base titration, the pH at the equivalence point is 7.
2. Weak Acid - Strong Base Titration
This scenario is more complex because the conjugate base of the weak acid hydrolyzes, affecting the pH. Let's consider the titration of 25.00 mL of 0.100 M acetic acid (CH₃COOH, Ka = 1.8 x 10⁻⁵) with 0.100 M NaOH.
Steps:
- Determine the moles of acid: Moles CH₃COOH = (0.100 mol/L) * (0.02500 L) = 0.00250 mol
- Determine the volume of base at equivalence: Similar to the previous example, 25.00 mL of NaOH is needed.
- Calculate the concentration of the conjugate base: At the equivalence point, all the acetic acid has reacted to form acetate ions (CH₃COO⁻). The concentration of acetate is: [CH₃COO⁻] = 0.00250 mol / 0.05000 L = 0.0500 M
- Use the Kb expression to calculate the hydroxide ion concentration: Kb = Kw/Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰ Kb = [OH⁻][CH₃COOH] / [CH₃COO⁻] (We can approximate [OH⁻] ≈ [CH₃COOH]) 5.6 x 10⁻¹⁰ = x² / 0.0500 x = [OH⁻] = √(5.6 x 10⁻¹⁰ * 0.0500) = 5.3 x 10⁻⁶ M
- Calculate the pOH: pOH = -log[OH⁻] = -log(5.3 x 10⁻⁶) ≈ 5.28
- Calculate the pH: pH = 14 - pOH = 14 - 5.28 ≈ 8.72
Therefore, the pH at the equivalence point is approximately 8.72. Note: The approximation used in step 4 is valid because Kb is very small.
3. Strong Acid - Weak Base Titration
This is analogous to the weak acid-strong base titration. The conjugate acid of the weak base hydrolyzes, lowering the pH. Let's consider the titration of 25.00 mL of 0.100 M HCl with 0.100 M ammonia (NH₃, Kb = 1.8 x 10⁻⁵).
Steps:
- Determine moles of acid: Moles HCl = 0.00250 mol (as before)
- Determine volume of base at equivalence: 25.00 mL of NH₃ is needed.
- Calculate the concentration of the conjugate acid: At the equivalence point, all the ammonia has reacted to form ammonium ions (NH₄⁺). The concentration of ammonium ions is 0.0500 M.
- Use the Ka expression to calculate the hydronium ion concentration: Ka = Kw/Kb = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰ Ka = [H⁺][NH₃] / [NH₄⁺] (We approximate [H⁺] ≈ [NH₃]) 5.6 x 10⁻¹⁰ = x² / 0.0500 x = [H⁺] = √(5.6 x 10⁻¹⁰ * 0.0500) = 5.3 x 10⁻⁶ M
- Calculate the pH: pH = -log[H⁺] = -log(5.3 x 10⁻⁶) ≈ 5.28
The pH at the equivalence point is approximately 5.28.
4. Weak Acid - Weak Base Titration
This is the most complex case. The pH at the equivalence point depends on the relative strengths of the acid and base, and a precise calculation often requires solving a quadratic equation or using an iterative approach. A simplified approach involves considering the relative Ka and Kb values. If Ka and Kb are comparable, the pH will be near 7. If the acid is significantly stronger (Ka >> Kb), the pH will be slightly acidic. If the base is significantly stronger (Kb >> Ka), the pH will be slightly basic.
Choosing the Right Indicator
The choice of indicator for a titration depends on the pH change at the equivalence point. Indicators are weak acids or bases that change color over a specific pH range. An appropriate indicator should have a color change that encompasses the equivalence point.
- Strong Acid - Strong Base: Phenolphthalein (pH range 8.2-10.0) or bromothymol blue (pH range 6.0-7.6) are commonly used.
- Weak Acid - Strong Base: Phenolphthalein is often suitable.
- Strong Acid - Weak Base: Methyl orange (pH range 3.1-4.4) or methyl red (pH range 4.4-6.2) are often preferred.
- Weak Acid - Weak Base: Choosing an indicator is challenging and requires careful consideration of the pH change near the equivalence point.
Practical Considerations and Error Analysis
Accurate pH calculations at the equivalence point rely on precise measurements of volume and concentration. Errors can arise from:
- Imprecise measurements: Inaccurate burette readings or concentration errors can significantly affect the results.
- Incomplete reaction: If the reaction doesn't go to completion, the calculated pH will be inaccurate.
- Temperature effects: The equilibrium constants (Ka and Kb) are temperature-dependent.
- Ionic strength: High ionic strength can alter the activity coefficients of ions, affecting the pH.
Conclusion
Calculating the pH at the equivalence point is a fundamental skill in analytical chemistry. Understanding the different scenarios and employing appropriate calculation methods is crucial for accurate analysis. Careful consideration of the acid-base properties, selection of appropriate indicators, and awareness of potential errors are essential for achieving reliable results in titration experiments. This detailed explanation empowers you to tackle various titration problems confidently and accurately predict the pH at the equivalence point under different conditions. Remember that practice is key; working through numerous examples will solidify your understanding of these concepts.
Latest Posts
Latest Posts
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Calculating The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
