Can The Atomic Mass Of An Element Vary
Kalali
Mar 16, 2025 · 5 min read
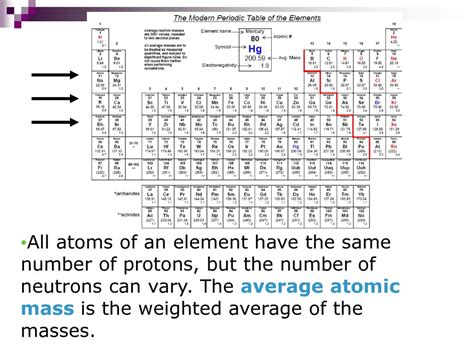
Table of Contents
Can the Atomic Mass of an Element Vary?
The atomic mass of an element, a fundamental concept in chemistry, isn't as fixed as one might initially assume. While periodic tables list a single atomic mass for each element, this is actually a weighted average reflecting the natural abundance of different isotopes. Understanding this nuanced concept is key to grasping the true nature of atomic mass and its variations. This article delves deep into the reasons why atomic mass can vary, exploring isotopes, mass spectrometry, and the implications of these variations in various scientific fields.
Understanding Isotopes: The Foundation of Variable Atomic Mass
The seemingly simple atomic mass listed for each element on the periodic table actually masks a complex reality. The atomic mass represents the average mass of all the naturally occurring isotopes of that element. Isotopes are atoms of the same element that possess the same number of protons (defining the element) but differ in the number of neutrons. This difference in neutron number directly impacts the atom's mass.
Neutron's Role in Isotopic Variation
Neutrons, unlike protons, do not contribute to an element's chemical properties. However, they significantly affect the atom's mass. Adding or subtracting neutrons creates different isotopes of the same element. For instance, carbon-12 (¹²C), carbon-13 (¹³C), and carbon-14 (¹⁴C) are all isotopes of carbon. They all have six protons, but they possess six, seven, and eight neutrons, respectively. This results in different atomic masses for each isotope.
Abundance and Weighted Average
The atomic mass listed in the periodic table isn't the mass of a single isotope; it's a weighted average of the masses of all naturally occurring isotopes of that element. The weighting factor is the relative abundance of each isotope in nature. Elements with a higher abundance of heavier isotopes will have a higher average atomic mass.
For example, chlorine (Cl) has two main isotopes: chlorine-35 (³⁵Cl) and chlorine-37 (³⁷Cl). ³⁵Cl is much more abundant (approximately 75%) than ³⁷Cl (approximately 25%). Therefore, the average atomic mass of chlorine, approximately 35.45 amu (atomic mass units), reflects this abundance-weighted average of the two isotopic masses.
Measuring Isotopic Abundance: Mass Spectrometry
Determining the precise atomic mass of an element and the relative abundance of its isotopes requires sophisticated techniques. Mass spectrometry is the primary method used for this purpose. This technique separates ions based on their mass-to-charge ratio.
The Mass Spectrometry Process
In mass spectrometry, a sample of the element is ionized, meaning electrons are removed, creating charged atoms or molecules (ions). These ions are then accelerated through a magnetic field, which separates them based on their mass-to-charge ratio. The heavier ions are deflected less than the lighter ions. A detector then measures the abundance of each ion, providing data on the relative abundance of each isotope.
Data Interpretation and Atomic Mass Calculation
The data from mass spectrometry yields a mass spectrum, a graph showing the relative abundance of each isotope plotted against its mass-to-charge ratio. From this spectrum, scientists can accurately determine the relative abundance of each isotope and calculate the weighted average atomic mass, reflecting the element's natural isotopic composition.
Factors Affecting Isotopic Abundance
The relative abundance of isotopes isn't constant across all samples of an element. Several factors can influence this abundance:
-
Geological Location: The isotopic composition of an element can vary slightly depending on its geological origin. Different rock formations can have varying ratios of isotopes due to processes like radioactive decay and geological fractionation.
-
Biological Processes: Biological processes can also fractionate isotopes. For example, plants preferentially take up lighter isotopes of carbon (¹²C) over heavier isotopes (¹³C). This leads to variations in the carbon isotope ratio in different organisms.
-
Artificial Enrichment: Human activities, such as nuclear reactions, can also alter the isotopic composition of elements. For instance, the production of enriched uranium for nuclear reactors leads to an artificial increase in the abundance of ²³⁵U compared to its natural abundance.
Implications of Variable Atomic Mass
The variations in atomic mass, while subtle for many elements, have significant implications across various scientific disciplines:
Geochemistry and Geology
Isotope ratios are powerful tools in geochemistry and geology. Variations in isotope ratios can help scientists understand geological processes, trace the origins of rocks and minerals, and even date geological formations. For example, radioactive isotopes, like uranium and potassium, are used for radiometric dating, enabling the determination of the age of rocks and fossils.
Environmental Science
Isotope ratios are extensively utilized in environmental science. For instance, studying the ratios of stable isotopes of carbon and oxygen in water can provide insights into climate change and water cycle processes. Analyzing isotope ratios in pollutants can help trace the sources of contamination.
Forensic Science and Archaeology
Isotope ratios can be used as forensic tools. Analyzing the isotopic composition of materials found at a crime scene, such as hair or bone, can provide clues about the origin and diet of individuals. Similarly, in archaeology, isotope analysis helps reveal dietary patterns and migration routes of ancient populations.
Medicine and Biology
Stable isotopes are used as tracers in medical and biological research. These isotopes, which are non-radioactive, can be incorporated into molecules to track their movement and metabolism within the body. This technique helps study biological processes, diagnose diseases, and develop new treatments.
Nuclear Physics and Chemistry
In nuclear physics and chemistry, understanding the properties of isotopes is crucial. Different isotopes of an element can have dramatically different nuclear properties, leading to applications in nuclear reactors, nuclear medicine (radioisotopes), and nuclear weapons technology.
Conclusion: A Deeper Understanding of Atomic Mass
The atomic mass of an element, far from being a static quantity, reflects a dynamic interplay of isotopic abundance and variation. Understanding this fundamental principle is essential for a multitude of scientific and technological advancements. Mass spectrometry provides the crucial tool for determining these isotopic abundances, allowing scientists to delve into the intricate details of isotopic variations and their far-reaching implications across diverse scientific disciplines, from geology to medicine. The variations in atomic mass, while often small, unlock crucial insights into Earth's history, environmental processes, biological systems, and the fundamental nature of matter itself. Further research and technological advancements continue to refine our understanding of isotopic variations, expanding the applications of this pivotal concept in numerous fields.
Latest Posts
Latest Posts
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 500
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Can The Atomic Mass Of An Element Vary . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
