Click On The Beaker That Shows The Brønsted-lowry Base.
Kalali
Mar 27, 2025 · 6 min read
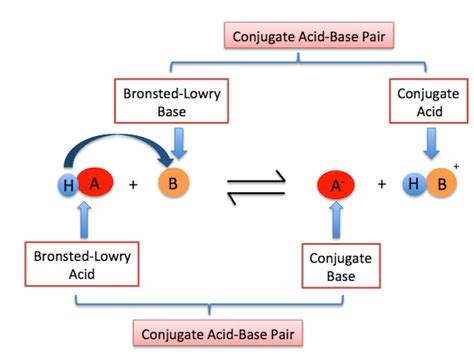
Table of Contents
Click on the Beaker that Shows the Brønsted-Lowry Base: A Deep Dive into Acid-Base Chemistry
Understanding acid-base chemistry is fundamental to numerous scientific disciplines, from biochemistry and environmental science to materials science and medicine. While several acid-base theories exist (Arrhenius, Lewis, and Brønsted-Lowry), the Brønsted-Lowry definition provides a particularly versatile and widely applicable framework for understanding acid-base reactions. This article will explore the Brønsted-Lowry definition, focusing on identifying Brønsted-Lowry bases and differentiating them from other types of bases. We'll delve into examples, mechanisms, and applications to solidify your understanding.
Defining Brønsted-Lowry Acids and Bases
Unlike the Arrhenius definition, which restricts acids and bases to aqueous solutions, the Brønsted-Lowry theory broadens the scope considerably. According to Brønsted-Lowry theory:
-
A Brønsted-Lowry acid is a substance that donates a proton (H⁺). This proton donation is the key characteristic. The acid doesn't necessarily need to be dissolved in water to exhibit acidic behavior.
-
A Brønsted-Lowry base is a substance that accepts a proton (H⁺). This proton acceptance is crucial. The base will have a lone pair of electrons to attract and bind to the proton.
This seemingly simple definition allows for a much wider range of substances to be classified as acids and bases, going beyond the limitations of the Arrhenius definition. This expansion is crucial for understanding acid-base reactions in non-aqueous solvents and in gaseous phases.
Key Differences from Arrhenius Definition
The Arrhenius definition defines acids as substances that produce H⁺ ions in water and bases as substances that produce OH⁻ ions in water. The limitations are obvious: this definition is exclusively applicable to aqueous solutions. The Brønsted-Lowry definition transcends this limitation by focusing on proton transfer, making it significantly more versatile. For example, ammonia (NH₃) acts as a base by accepting a proton, even in non-aqueous solutions, a concept not encompassed by the Arrhenius theory.
Identifying Brønsted-Lowry Bases: A Practical Approach
Identifying a Brønsted-Lowry base involves looking for molecules or ions with a lone pair of electrons capable of accepting a proton. Let's break down some strategies:
1. Look for Lone Pairs of Electrons
The presence of lone pairs of electrons is the most crucial indicator. These electron pairs can form a coordinate covalent bond with a proton (H⁺). Molecules or ions with nitrogen, oxygen, sulfur, or halogens often possess lone pairs and are likely to be Brønsted-Lowry bases.
Examples:
- Ammonia (NH₃): The nitrogen atom has a lone pair, readily accepting a proton to form the ammonium ion (NH₄⁺).
- Water (H₂O): Oxygen has two lone pairs, making water amphoteric (it can act as both an acid and a base).
- Hydroxide ion (OH⁻): The oxygen atom has a lone pair, strongly attracting a proton.
2. Consider the Molecular Structure
The molecular structure influences the availability of lone pairs and their ability to accept a proton. Steric hindrance (bulky groups around the lone pair) can decrease the base's strength by hindering proton access.
Examples:
- Tertiary amines are generally weaker bases than primary amines: The bulky alkyl groups around the nitrogen atom in tertiary amines sterically hinder proton approach.
- Cyclic amines: The ring structure can affect the basicity depending on the ring size and substituents.
3. Analyze the Conjugate Acid
Every Brønsted-Lowry base has a conjugate acid formed after accepting a proton. Understanding the stability of the conjugate acid can help predict the base's strength. A more stable conjugate acid implies a stronger base.
Examples:
- The conjugate acid of ammonia (NH₄⁺) is relatively stable, making ammonia a reasonably strong base.
- The conjugate acid of a weak base will be a relatively strong acid.
Examples of Brønsted-Lowry Bases in Action
Let's look at some reactions to illustrate the concept:
1. Reaction of Ammonia with Water:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
In this reaction, ammonia (NH₃) acts as a Brønsted-Lowry base by accepting a proton from water (which acts as a Brønsted-Lowry acid). Water donates a proton, forming the hydroxide ion (OH⁻), and ammonia accepts the proton, forming the ammonium ion (NH₄⁺).
2. Reaction of Chloride Ion with Water:
Cl⁻(aq) + H₂O(l) ⇌ HCl(aq) + OH⁻(aq)
While less pronounced, the chloride ion can also act as a Brønsted-Lowry base. It is a very weak base due to the low electronegativity of chloride and the resulting relatively low attraction for the proton.
3. Reaction of Acetate Ion with Water:
CH₃COO⁻(aq) + H₂O(l) ⇌ CH₃COOH(aq) + OH⁻(aq)
Acetate ion acts as a Brønsted-Lowry base in this reaction, accepting a proton from water and generating acetic acid and hydroxide ion. The strength of acetate ion as a base stems from the resonance stabilization of the acetate anion which makes it a better proton acceptor.
Factors Affecting the Strength of Brønsted-Lowry Bases
The strength of a Brønsted-Lowry base depends on several factors:
-
Electronegativity: Atoms with lower electronegativity tend to be stronger bases because they hold onto their electrons less tightly, making them more likely to share them with a proton.
-
Size of the Atom: Larger atoms are generally weaker bases because the electron density is spread over a larger volume, making it less concentrated and less readily available for proton acceptance.
-
Resonance: Resonance structures can stabilize the base or its conjugate acid, influencing the base strength. Increased resonance stabilization generally strengthens the base.
-
Inductive Effects: Electron-donating groups enhance base strength while electron-withdrawing groups weaken it.
Brønsted-Lowry Bases in Everyday Life and Applications
Brønsted-Lowry bases are prevalent in various aspects of our lives and have numerous applications:
-
Pharmaceuticals: Many medications are bases and exert their effects by interacting with body components via proton transfer.
-
Agriculture: Many fertilizers contain compounds that act as Brønsted-Lowry bases.
-
Cleaning Products: Many household cleaners are basic solutions, often utilizing the properties of Brønsted-Lowry bases to dissolve fats and oils.
-
Industrial Processes: Numerous industrial processes involve acid-base reactions, using Brønsted-Lowry bases in catalysis and other roles.
Conclusion: Mastering the Concept of Brønsted-Lowry Bases
Understanding the Brønsted-Lowry definition of bases is paramount for grasping acid-base chemistry's broader context. By focusing on proton acceptance and examining the factors influencing base strength, you'll be able to confidently identify Brønsted-Lowry bases in diverse chemical systems. This knowledge forms a strong foundation for further explorations in organic chemistry, biochemistry, and numerous related fields. Remember to look for those lone pairs and consider the consequences of proton acceptance – the stability of the conjugate acid – to effectively "click on the beaker" that shows the Brønsted-Lowry base. The ability to correctly identify and understand these bases is crucial to unlocking the intricate world of acid-base reactions.
Latest Posts
Latest Posts
-
32 In Is How Many Feet
Mar 31, 2025
-
How Many Inches Is 138 Cm
Mar 31, 2025
-
What Do All Of The Planets Have In Common
Mar 31, 2025
-
180 Inches Is How Many Feet
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Click On The Beaker That Shows The Brønsted-lowry Base. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
