Compare And Contrast The Two Types Of Fermentation
Kalali
Mar 31, 2025 · 6 min read
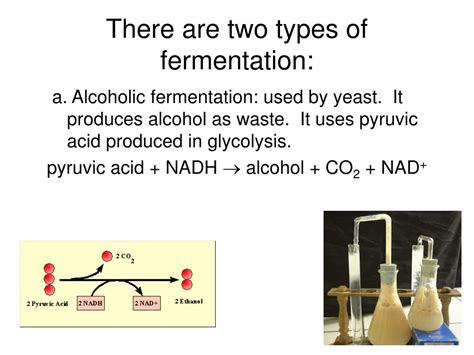
Table of Contents
Comparing and Contrasting the Two Main Types of Fermentation: Lactic Acid and Alcoholic
Fermentation, a metabolic process that extracts energy from carbohydrates, plays a crucial role in various industries, from food production to biofuel generation. While seemingly simple, the intricacies of fermentation are fascinating, with different types yielding unique products and exhibiting distinct metabolic pathways. This article delves into the comparison and contrast of the two primary types of fermentation: lactic acid fermentation and alcoholic fermentation. Understanding their differences is key to appreciating the vast applications of these ancient processes.
Lactic Acid Fermentation: The Sour Powerhouse
Lactic acid fermentation, a cornerstone of many food preservation techniques, is an anaerobic process (occurring without oxygen) whereby glucose is converted into lactic acid. This metabolic pathway is primarily employed by certain bacteria (lactobacilli and streptococci) and some animal cells (muscle cells during strenuous activity). The simplicity of the process and the relative ease of cultivation of lactic acid bacteria have made it a mainstay in food production for millennia.
The Biochemical Mechanism: A Step-by-Step Look
The core reaction of lactic acid fermentation starts with glycolysis, the breakdown of glucose (a six-carbon sugar) into two molecules of pyruvate (a three-carbon compound). This initial stage generates a net gain of two ATP (adenosine triphosphate) molecules, the cell's primary energy currency. Crucially, glycolysis doesn't require oxygen.
In lactic acid fermentation, pyruvate is then directly reduced (gains electrons) to form lactic acid. This reduction step utilizes the NADH (nicotinamide adenine dinucleotide) molecules produced during glycolysis. NADH acts as an electron carrier, transferring electrons to pyruvate. The regeneration of NAD+ from NADH is essential; without it, glycolysis would halt due to a lack of electron acceptors. This recycling of NAD+ is what allows for the continuous production of ATP in the absence of oxygen.
Products and Applications: From Yogurt to Sauerkraut
The lactic acid produced through this process is responsible for the characteristic sour taste of many fermented foods. This acidity also acts as a natural preservative, inhibiting the growth of spoilage organisms. Some key applications of lactic acid fermentation include:
-
Yogurt and Kefir: These dairy products are created through the fermentation of milk by lactic acid bacteria, resulting in a thicker texture and tangy flavor. The specific strains of bacteria used dictate the final product's characteristics.
-
Sauerkraut and Pickles: Vegetables like cabbage and cucumbers are fermented in a brine solution, allowing lactic acid bacteria to thrive and produce lactic acid. This process extends shelf life and enhances flavor.
-
Silage: Lactic acid fermentation is crucial in preserving livestock feed (silage). The process converts sugars in plants into lactic acid, inhibiting spoilage and creating a nutritious feed source.
-
Some types of cheese: Lactic acid bacteria play a critical role in cheesemaking, influencing flavor, texture, and preservation.
-
Industrial applications: Lactic acid itself finds widespread use in various industries, including food additives, pharmaceuticals, and biodegradable plastics.
Advantages and Disadvantages: Weighing the Pros and Cons
Advantages:
- Simplicity: The process is relatively straightforward, requiring minimal equipment and expertise.
- Preservation: The resulting lactic acid acts as a natural preservative, extending the shelf life of food products.
- Nutritional benefits: Fermentation can enhance the bioavailability of certain nutrients in food.
- Sustainable: The process often utilizes readily available substrates, making it a relatively sustainable option.
Disadvantages:
- Limited energy yield: Lactic acid fermentation produces only a small amount of ATP (2 molecules per glucose molecule) compared to aerobic respiration.
- Acid buildup: The accumulation of lactic acid can cause undesirable changes in taste and texture in some products.
- Potential for undesirable bacterial growth: If proper hygiene isn't maintained, unwanted bacteria can contaminate the fermentation process.
Alcoholic Fermentation: The Buzzworthy Process
Alcoholic fermentation, another anaerobic process, converts glucose into ethanol (ethyl alcohol) and carbon dioxide. This process is primarily carried out by yeasts, single-celled fungi. Unlike lactic acid fermentation, alcoholic fermentation involves a more complex series of reactions.
The Biochemical Mechanism: Beyond Glycolysis
As with lactic acid fermentation, alcoholic fermentation begins with glycolysis, yielding two molecules of pyruvate and two ATP. However, the fate of pyruvate differs significantly. Instead of being directly reduced to lactic acid, pyruvate undergoes a decarboxylation reaction, losing a carbon dioxide molecule to form acetaldehyde. This reaction is catalyzed by the enzyme pyruvate decarboxylase.
Acetaldehyde is then reduced by NADH to form ethanol. Similar to lactic acid fermentation, the regeneration of NAD+ is crucial for the continuation of glycolysis. This ensures the ongoing production of ATP, even in the absence of oxygen.
Products and Applications: From Wine to Beer to Biofuel
The ethanol produced through alcoholic fermentation is the primary intoxicating agent in alcoholic beverages. The carbon dioxide produced is responsible for the effervescence in many fermented drinks. Here are some of the key applications:
-
Winemaking: Grapes are crushed and fermented by yeasts, converting their sugars into ethanol and carbon dioxide. Different yeast strains and grape varieties contribute to the unique characteristics of various wines.
-
Beer brewing: Grains (barley, wheat, etc.) are malted and fermented by yeasts, producing beer with varying alcohol content and flavor profiles. Hops are often added for bitterness and aroma.
-
Breadmaking: Yeasts are used to leaven bread dough. The carbon dioxide produced during fermentation causes the dough to rise, while the ethanol evaporates during baking.
-
Biofuel production: Alcoholic fermentation is used to produce bioethanol, a renewable fuel source. Various plant materials (e.g., corn, sugarcane) can be used as substrates.
-
Other food applications: Alcoholic fermentation plays a role in the production of certain foods like kimchi and certain types of sourdough bread.
Advantages and Disadvantages: A Balanced Perspective
Advantages:
- High ethanol yield: Alcoholic fermentation can yield a significant amount of ethanol, making it valuable for biofuel production.
- Flavor enhancement: The production of ethanol and other byproducts can contribute to the complex flavors of alcoholic beverages and certain foods.
- Versatile substrates: A range of plant materials can serve as substrates for alcoholic fermentation.
Disadvantages:
- Sensitivity to conditions: Yeasts involved in alcoholic fermentation are sensitive to changes in temperature, pH, and nutrient availability.
- Potential for contamination: Unwanted microorganisms can contaminate the fermentation process, affecting product quality.
- Ethanol toxicity: High concentrations of ethanol can be toxic to humans and other organisms.
A Direct Comparison: Lactic Acid vs. Alcoholic Fermentation
| Feature | Lactic Acid Fermentation | Alcoholic Fermentation |
|---|---|---|
| Organism | Bacteria (lactobacilli, streptococci), some animal cells | Yeasts |
| End Product | Lactic acid | Ethanol and carbon dioxide |
| Oxygen | Anaerobic (no oxygen required) | Anaerobic (no oxygen required) |
| Energy Yield | Low (2 ATP per glucose) | Low (2 ATP per glucose) |
| Applications | Food preservation (yogurt, sauerkraut, pickles), silage | Alcoholic beverages (wine, beer), biofuel, breadmaking |
| Taste Profile | Sour | Variable, depending on the substrate and other factors |
| Preservation | High (due to lactic acid's antimicrobial properties) | Lower (depends on ethanol concentration and other factors) |
Conclusion: Two Sides of the Same Coin
Lactic acid and alcoholic fermentation, while both anaerobic processes yielding relatively low ATP, are distinct pathways with unique applications. Lactic acid fermentation's focus on acid production makes it ideal for food preservation, while alcoholic fermentation's ethanol output plays a key role in beverage production and biofuel generation. Understanding the biochemical mechanisms and specific applications of each type is crucial for appreciating the profound impact these processes have had on human history and continue to have on modern industries. From the tangy bite of yogurt to the intoxicating allure of wine, the legacy of these ancient processes is evident in the richness and diversity of our food and beverage landscape.
Latest Posts
Latest Posts
-
22 5 Out Of 30 As A Percentage
Apr 01, 2025
-
How Do You Write 2 As A Decimal
Apr 01, 2025
-
How Many Inches Are In 1 1 2
Apr 01, 2025
-
What Percent Of 600 Is 3
Apr 01, 2025
-
How Tall Is 24 Inches In Feet
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Compare And Contrast The Two Types Of Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
