Difference Between A Reactant And A Product
Kalali
Mar 19, 2025 · 6 min read
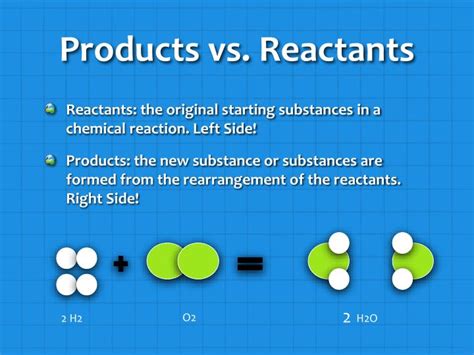
Table of Contents
The Fundamental Difference Between Reactants and Products in Chemical Reactions
Understanding the difference between reactants and products is fundamental to grasping the core concepts of chemistry. While seemingly simple, this distinction underpins our comprehension of chemical reactions, their mechanisms, and their applications across various fields, from medicine to materials science. This comprehensive guide delves deep into the nature of reactants and products, exploring their roles, identifying key distinctions, and illustrating these concepts with real-world examples.
What are Reactants?
Reactants are the starting materials in a chemical reaction. These are the substances that undergo a chemical change, transforming into something new. Think of them as the ingredients in a culinary recipe. Before the reaction begins, reactants exist independently, possessing their own unique chemical and physical properties. During the reaction, the chemical bonds within the reactants are broken, and new bonds are formed, leading to the creation of products. Reactants are typically found on the left-hand side of a chemical equation.
Key Characteristics of Reactants:
- Undergo chemical change: Reactants are actively involved in the chemical process, experiencing a transformation in their chemical composition.
- Chemical bonds broken: Existing bonds within reactant molecules are severed during the reaction.
- Located on the left: In a chemical equation, reactants are always written on the left side of the arrow.
- Determines the outcome: The nature and quantities of reactants significantly influence the products formed and the reaction's overall outcome.
- Can be elements or compounds: Reactants can be individual elements (like hydrogen or oxygen) or complex chemical compounds (like glucose or sodium chloride).
Examples of Reactants:
- Combustion of Methane: In the burning of methane (CH₄), methane and oxygen (O₂) are the reactants.
- CH₄ + 2O₂ → CO₂ + 2H₂O
- Formation of Water: In the synthesis of water, hydrogen (H₂) and oxygen (O₂) act as reactants.
- 2H₂ + O₂ → 2H₂O
- Neutralization Reaction: In the neutralization of an acid with a base, the acid and base are the reactants. For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
- HCl + NaOH → NaCl + H₂O
What are Products?
Products are the newly formed substances that result from a chemical reaction. They are the outcome of the chemical transformation of the reactants. Continuing the culinary analogy, products are the finished dish – the result of combining and altering the ingredients (reactants). Products possess unique properties that differ from those of the original reactants. They are typically found on the right-hand side of a chemical equation.
Key Characteristics of Products:
- Result of chemical change: Products are formed as a direct consequence of the chemical reaction.
- New chemical bonds formed: New chemical bonds are created during the reaction to form the product molecules.
- Located on the right: In a chemical equation, products are always written on the right side of the arrow.
- Properties differ from reactants: Products often have significantly different physical and chemical properties compared to the starting reactants.
- Can be elements or compounds: Similar to reactants, products can be elements or compounds.
Examples of Products:
- Combustion of Methane: In the burning of methane, carbon dioxide (CO₂) and water (H₂O) are the products.
- CH₄ + 2O₂ → CO₂ + 2H₂O
- Formation of Water: Water (H₂O) is the product of the reaction between hydrogen and oxygen.
- 2H₂ + O₂ → 2H₂O
- Neutralization Reaction: In the neutralization of HCl and NaOH, sodium chloride (NaCl) and water (H₂O) are the products.
- HCl + NaOH → NaCl + H₂O
Distinguishing Reactants and Products: A Deeper Dive
The fundamental difference between reactants and products lies in their temporal relationship within a chemical reaction. Reactants are present before the reaction occurs, while products are formed after the reaction has taken place. This temporal distinction is crucial for understanding the flow of the reaction and predicting its outcome.
Chemical Equations: Chemical equations provide a concise representation of a chemical reaction, clearly delineating reactants and products. The arrow (→) signifies the transformation from reactants to products. The reactants are always written to the left of the arrow, and the products are always written to the right. The equation must be balanced, meaning the number of atoms of each element is the same on both sides of the arrow, reflecting the law of conservation of mass.
Reaction Mechanisms: Understanding reaction mechanisms provides a deeper insight into the stepwise transformation of reactants into products. These mechanisms detail the individual steps involved, including intermediate species formed during the transition. This intricate level of analysis reveals the specific bond breaking and bond forming processes underlying the overall reaction.
Stoichiometry: Stoichiometry is the quantitative aspect of chemical reactions, determining the relative amounts of reactants and products involved. By using stoichiometric calculations, we can predict the amount of product formed from a given quantity of reactants, or vice versa. This is crucial in industrial processes and laboratory experiments for efficient and precise control of reactions.
Reversibility and Equilibrium: While many reactions proceed essentially to completion, others are reversible. In reversible reactions, the products can react to reform the reactants. When the rates of the forward and reverse reactions become equal, a state of equilibrium is reached, with a dynamic balance between reactants and products.
The Importance of Reactants and Products in Various Fields
The distinction between reactants and products has far-reaching implications across numerous fields:
Medicine: Drug discovery and development heavily rely on understanding chemical reactions. The desired therapeutic effect of a drug often stems from its interaction with specific molecules in the body, acting as a reactant in a biochemical process. Understanding the products of these reactions is crucial for assessing drug efficacy, side effects, and metabolism.
Materials Science: The creation of new materials, from polymers to ceramics, hinges on carefully controlling chemical reactions. The properties of the final product are directly related to the starting reactants and the conditions under which they react. For example, the properties of steel are greatly influenced by the type and amount of carbon used as a reactant during its production.
Environmental Science: Understanding chemical reactions is vital for managing environmental pollution. The reactants involved in pollution processes and their resultant products are critical factors for developing effective remediation strategies. For instance, understanding the reactions leading to acid rain involves analyzing the reactants (gases like sulfur dioxide and nitrogen oxides) and their resulting products (acidic precipitation).
Food Science: Food chemistry heavily relies on understanding chemical reactions. The taste, texture, and preservation of food are all influenced by chemical changes occurring during preparation and storage. For example, the browning of bread during baking involves complex reactions between carbohydrates and amino acids, where reactants are transformed into products contributing to flavor and texture.
Conclusion
The distinction between reactants and products is a fundamental cornerstone of chemistry. Understanding their roles, characteristics, and relationships within chemical reactions is essential for comprehending a wide array of phenomena across various scientific disciplines. From synthesizing new materials to developing life-saving drugs, the knowledge of reactants and products is a powerful tool for innovation and problem-solving. The accurate representation of these components in balanced chemical equations remains a cornerstone of chemical communication and accurate quantitative predictions. By thoroughly understanding this fundamental concept, one gains a strong foundation for further exploration of the fascinating world of chemical reactions and their impact on our lives.
Latest Posts
Latest Posts
-
Is Sour Taste A Physical Property
Mar 19, 2025
-
How Many Kilos Are 20 Pounds
Mar 19, 2025
-
11 Out Of 30 As A Percentage
Mar 19, 2025
-
Cuanto Es 92 Grados Fahrenheit En Centigrados
Mar 19, 2025
-
How To Get Magnitude Of Force
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Difference Between A Reactant And A Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
