Does Carbon Have 4 Valence Electrons
Kalali
Mar 26, 2025 · 6 min read
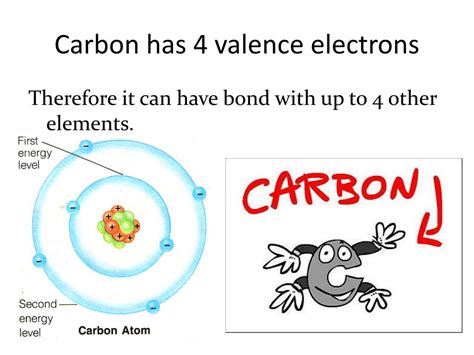
Table of Contents
Does Carbon Have 4 Valence Electrons? Unraveling the Chemistry of Carbon
Carbon, the backbone of life and a cornerstone of organic chemistry, possesses a unique electronic structure that dictates its remarkable versatility. A central question frequently arises: does carbon have 4 valence electrons? The answer, unequivocally, is yes. This fundamental characteristic underpins carbon's ability to form an astonishing array of molecules, driving the complexity and diversity we see in the organic world and beyond. This article will delve into the reasons behind this, exploring carbon's electronic configuration, its bonding behavior, and the consequences of having four valence electrons.
Understanding Valence Electrons
Before diving into carbon's specific case, let's establish a clear understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they are the ones involved in chemical bonding – the formation of links between atoms to create molecules. The number of valence electrons an atom possesses significantly determines its reactivity and the types of bonds it can form.
Atoms strive for stability, often achieved by having a full outermost shell. This often translates to eight electrons (the octet rule), though there are exceptions, particularly for smaller atoms. Atoms achieve this stability by either gaining, losing, or sharing electrons with other atoms.
Carbon's Electronic Configuration and Valence Electrons
Carbon's atomic number is 6, meaning it has six protons and six electrons. Its electronic configuration is 1s²2s²2p². Let's break this down:
- 1s²: Two electrons occupy the lowest energy level (n=1), filling the 1s orbital.
- 2s²: Two electrons occupy the next energy level (n=2), filling the 2s orbital.
- 2p²: Two electrons occupy the 2p sublevel, which consists of three 2p orbitals (2px, 2py, 2pz). Each 2p orbital can hold up to two electrons.
Crucially, the outermost shell (n=2) contains four electrons (2s²2p²). These are the valence electrons. It's these four electrons that participate in the formation of chemical bonds with other atoms.
Carbon's Bonding Behavior: The Power of Four Valence Electrons
The presence of four valence electrons allows carbon to form a variety of strong covalent bonds. Covalent bonds involve the sharing of electrons between atoms. Because carbon needs four more electrons to achieve a stable octet, it typically forms four covalent bonds. This ability to form four bonds is responsible for carbon's exceptional versatility.
Types of Carbon Bonds
Carbon can form single, double, and triple bonds. These variations arise from the different ways carbon can share its four valence electrons:
- Single bonds: Carbon shares one electron with each of four other atoms, resulting in four single bonds (e.g., methane, CH₄).
- Double bonds: Carbon shares two electrons with one atom and one electron each with two other atoms, resulting in one double bond and two single bonds (e.g., ethene, C₂H₄).
- Triple bonds: Carbon shares three electrons with one atom and one electron with another atom, resulting in one triple bond and one single bond (e.g., ethyne, C₂H₂).
The diverse combination of single, double, and triple bonds allows carbon to form a vast array of complex molecules with diverse shapes and properties.
The Significance of Carbon's Four Valence Electrons
Carbon's four valence electrons are the reason behind its dominance in organic chemistry and its crucial role in the chemistry of life. This unique feature results in several key properties:
-
Tetrahedral Geometry: When carbon forms four single bonds, the bonds are arranged in a tetrahedral geometry. This maximizes the distance between the bonded atoms, minimizing repulsions and contributing to the stability of the molecule.
-
Chain Formation: Carbon atoms can bond to each other in long chains, creating linear, branched, or cyclic structures. This property is fundamental to the formation of polymers, long chains of repeating units, which are essential components of many materials, including plastics and biological macromolecules like DNA and proteins.
-
Ring Formation: Carbon atoms can also bond to each other to form rings, creating cyclic molecules with unique properties. These rings are present in numerous natural and synthetic compounds, including sugars, aromatic compounds, and many pharmaceuticals.
-
Isomerism: The ability of carbon to form multiple bonds and chains leads to the phenomenon of isomerism. Isomers are molecules with the same molecular formula but different structural arrangements, resulting in different properties. This feature adds immense diversity to the number of possible organic molecules.
Carbon's Role in Life and Beyond
The implications of carbon having four valence electrons extend far beyond the realm of theoretical chemistry. It's the foundation of:
-
Organic Molecules: Carbon is the backbone of all organic molecules, the molecules that constitute living organisms. Carbohydrates, lipids, proteins, and nucleic acids – the essential building blocks of life – are all based on carbon skeletons.
-
Biomolecules: The complex three-dimensional structures of biomolecules, like enzymes and antibodies, are dependent on carbon's ability to form diverse bonds and chains. The specificity and functionality of these molecules are directly related to their carbon-based structures.
-
Materials Science: Carbon's versatility is exploited in materials science to create novel materials with desired properties. Diamonds, graphite, fullerenes, and carbon nanotubes are all allotropes of carbon, showcasing the diverse range of materials possible from this single element. Each allotrope possesses unique properties depending on the arrangement of the carbon atoms and the types of bonds they form.
Beyond the Octet Rule: Exceptions and Complications
While the octet rule is a useful guideline, there are exceptions, especially when dealing with elements beyond the second row of the periodic table. Carbon itself exhibits some exceptions, particularly in certain organometallic compounds where carbon may have more or fewer than four bonds. These exceptions arise from the complexities of orbital hybridization and the involvement of d-orbitals in bonding. However, the fundamental principle that carbon possesses four valence electrons remains central to understanding its behavior in the vast majority of cases.
Conclusion: The Central Role of Four Valence Electrons
The answer to the question, "Does carbon have 4 valence electrons?" is definitively yes. This seemingly simple fact is the foundation of carbon's extraordinary versatility and its crucial role in both organic chemistry and the chemistry of life. Carbon's ability to form four covalent bonds, leading to diverse structures and properties, drives the complexity and richness of the chemical world around us. Understanding this fundamental characteristic is key to comprehending the vast and fascinating realm of carbon chemistry. From the simplest organic molecules to the most complex biomolecules and advanced materials, carbon's four valence electrons are the key to unlocking a world of possibilities.
Latest Posts
Latest Posts
-
How Big Is 70 Cm In Inches
Mar 29, 2025
-
6 Cups Is How Many Pints
Mar 29, 2025
-
How Many Liters Are In 500 Ml
Mar 29, 2025
-
1 5 Oz Is How Many Grams
Mar 29, 2025
-
38 Out Of 40 Is What Percent
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Does Carbon Have 4 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
