Does Hydrogen Have More Electrons Than Uranium
Kalali
Mar 11, 2025 · 5 min read
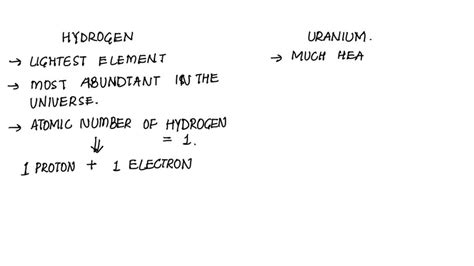
Table of Contents
Does Hydrogen Have More Electrons Than Uranium? Understanding Atomic Structure
The question, "Does hydrogen have more electrons than uranium?" might seem simple at first glance. However, a proper answer requires delving into the fundamental principles of atomic structure and the periodic table. The short answer is a resounding no. Uranium possesses significantly more electrons than hydrogen. Let's explore why.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Atoms, the basic building blocks of matter, are composed of three subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and defines its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
The arrangement of these particles, particularly the electrons, dictates an element's chemical properties and how it interacts with other elements.
Atomic Number and the Periodic Table
The periodic table is a crucial tool for understanding the relationship between elements. Each element is assigned an atomic number, representing the number of protons in its nucleus. This number is unique to each element and is arranged sequentially in the periodic table.
- Hydrogen (H): Atomic number 1. This means a hydrogen atom has one proton. In its neutral state, it also has one electron.
- Uranium (U): Atomic number 92. This indicates a uranium atom possesses 92 protons. In its neutral state, it also has 92 electrons.
Electron Shells and Orbitals
Electrons don't randomly orbit the nucleus; they occupy specific energy levels called electron shells. Each shell can hold a limited number of electrons. The shells are filled sequentially, starting with the lowest energy level closest to the nucleus. The first shell can hold up to two electrons, the second shell up to eight, and so on. Within each shell, electrons occupy orbitals, which represent regions of space where there's a high probability of finding an electron.
The electron configuration, which describes how electrons are distributed among the shells and orbitals, is crucial in determining an element's chemical behavior. For example, hydrogen's single electron in its first shell makes it highly reactive, while uranium's complex electron configuration leads to its unique chemical properties.
Why Uranium Has Significantly More Electrons
The vast difference in the number of electrons between hydrogen and uranium stems directly from their atomic numbers. Uranium's atomic number of 92 signifies that it has 92 protons in its nucleus, requiring 92 electrons to balance the positive charge and maintain electrical neutrality in a neutral uranium atom. Hydrogen, with its atomic number of 1, has only one proton and consequently one electron in its neutral state.
This fundamental difference in atomic structure results in a wide disparity in their physical and chemical properties. Hydrogen is a light, highly reactive gas, while uranium is a dense, radioactive metal.
Isotopes and Ions: Variations in Electron Count
While the number of electrons usually equals the number of protons in a neutral atom, there are exceptions:
- Isotopes: Atoms of the same element with the same number of protons but a different number of neutrons. Isotopes have the same number of electrons in their neutral state. For example, different isotopes of uranium will all have 92 electrons when neutral.
- Ions: Atoms that have gained or lost electrons, resulting in a net positive or negative charge. A positively charged ion (cation) has fewer electrons than protons, while a negatively charged ion (anion) has more electrons than protons. The number of electrons in an ion is different from the number of protons.
It's crucial to note that even though ions can have a different number of electrons than protons, the number of electrons in a uranium ion will still be far greater than the number of electrons in a hydrogen atom, even if the uranium atom has lost several electrons.
Comparing Hydrogen and Uranium: A Summary
| Feature | Hydrogen (H) | Uranium (U) |
|---|---|---|
| Atomic Number | 1 | 92 |
| Number of Protons | 1 | 92 |
| Number of Electrons (neutral atom) | 1 | 92 |
| Electron Shells | 1 | Multiple |
| Reactivity | High | Complex |
| Physical State | Gas | Solid |
| Radioactivity | Non-radioactive | Radioactive |
Beyond the Basics: Electron Configuration and Quantum Mechanics
A more detailed understanding of electron configuration requires delving into quantum mechanics. Quantum mechanics describes the behavior of electrons at the atomic level. Electrons don't simply orbit the nucleus in neat, circular paths; instead, they exist in orbitals defined by probability distributions. These orbitals are arranged in shells and subshells, with each subshell having a specific number of orbitals. The filling of these orbitals follows specific rules, leading to the unique electron configurations of different elements.
The electron configuration of uranium is far more complex than that of hydrogen, reflecting the presence of many more electrons distributed among numerous shells and subshells. This complexity is a significant factor in uranium's unique chemical and physical properties.
Conclusion: A Definitive No
In conclusion, hydrogen unequivocally does not have more electrons than uranium. Uranium, with its atomic number of 92, has 92 protons and, in its neutral state, 92 electrons. Hydrogen, with its atomic number of 1, has only one proton and one electron. The difference in electron count is vast, reflecting the fundamental differences in their atomic structures and resulting properties. While considering isotopes and ions can introduce variations in electron count, uranium will always possess a far greater number of electrons than hydrogen. This simple yet fundamental difference highlights the complexity and beauty of the periodic table and the world of atomic structure.
Latest Posts
Latest Posts
-
How Long To Heat Water In Microwave
Jul 18, 2025
-
40 Oz Of Water Is How Many Cups
Jul 18, 2025
-
How Many Eighths In A Quarter Pound
Jul 18, 2025
-
Can The Sine Of An Angle Ever Equal 2
Jul 18, 2025
-
How Many Months Is A Hundred Days
Jul 18, 2025
Related Post
Thank you for visiting our website which covers about Does Hydrogen Have More Electrons Than Uranium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.