Does Oxygen Lose Or Gain Electrons
Kalali
Mar 27, 2025 · 5 min read
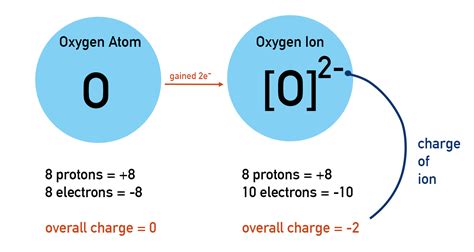
Table of Contents
Does Oxygen Lose or Gain Electrons? Understanding Oxidation and Reduction
Oxygen's role in chemical reactions is fundamentally linked to its electron behavior. The question, "Does oxygen lose or gain electrons?" is crucial to understanding its reactivity and its vital role in processes like respiration and combustion. The simple answer is that oxygen almost always gains electrons in chemical reactions. This behavior is the hallmark of its strong oxidizing power, a property with profound implications across various scientific fields. This article delves deep into the electronic structure of oxygen, its propensity to gain electrons, and the consequences of this behavior in different contexts.
Oxygen's Electronic Structure: The Foundation of its Reactivity
To understand why oxygen gains electrons, we must examine its electronic structure. Oxygen, with an atomic number of 8, possesses eight electrons. Its electronic configuration is 1s²2s²2p⁴. This means it has two electrons in the first shell (1s²) and six electrons in the second shell (2s²2p⁴). The outermost shell, the valence shell, contains six electrons. Atoms strive for a stable electron configuration, often achieving this by having a full outermost shell, which is usually eight electrons (the octet rule, although there are exceptions).
Oxygen, with its six valence electrons, is two electrons short of a full octet. This electron deficiency makes it highly reactive. Achieving a stable octet is energetically favorable for oxygen, and it readily accomplishes this by gaining two electrons. This gain of electrons is what defines oxygen's behavior as an oxidizing agent.
The Role of Electronegativity
Oxygen's high electronegativity significantly contributes to its electron-gaining tendency. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Oxygen boasts a high electronegativity value (3.44 on the Pauling scale), second only to fluorine. This high electronegativity means that when oxygen forms a bond with another atom, it strongly attracts the shared electrons towards itself, often to the point of effectively gaining them.
Oxidation: Oxygen's Signature Reaction
The process by which oxygen gains electrons is called reduction, while the simultaneous process by which another atom or molecule loses electrons is called oxidation. These two processes are always coupled; one cannot occur without the other. This coupled process is known as a redox reaction (reduction-oxidation reaction). In a redox reaction involving oxygen, oxygen is always the oxidizing agent, meaning it causes the oxidation of another substance while it itself is reduced.
Examples of Oxygen's Electron Gain
Let's consider some examples to illustrate oxygen's tendency to gain electrons:
-
Combustion: When a substance like methane (CH₄) burns in the presence of oxygen, the carbon and hydrogen atoms lose electrons (oxidation) while oxygen gains electrons (reduction). The overall reaction is: CH₄ + 2O₂ → CO₂ + 2H₂O. In this reaction, carbon goes from an oxidation state of -4 to +4, and hydrogen from +1 to +1. Oxygen's oxidation state changes from 0 to -2. This demonstrates the electron transfer from carbon and hydrogen to oxygen.
-
Respiration: Cellular respiration, the process that powers life, involves the oxidation of glucose (C₆H₁₂O₆) by oxygen. Glucose loses electrons (oxidation) while oxygen gains electrons (reduction), producing carbon dioxide (CO₂) and water (H₂O) as byproducts. The simplified equation is: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O. Again, oxygen's oxidation state changes from 0 to -2.
-
Rusting (Corrosion): The rusting of iron is another classic example of oxygen's oxidizing power. Iron reacts with oxygen and water to form iron oxide (rust), Fe₂O₃. Iron loses electrons (oxidation), while oxygen gains electrons (reduction). The reaction is: 4Fe + 3O₂ + 6H₂O → 4Fe(OH)₃.
Exceptions: Peroxides and Superoxides
While oxygen almost always gains electrons, there are exceptions. In certain circumstances, oxygen can exist in unusual oxidation states, such as in peroxides (e.g., hydrogen peroxide, H₂O₂) and superoxides (e.g., potassium superoxide, KO₂).
Peroxides: An Oxidation State of -1
In peroxides, oxygen has an oxidation state of -1. This means each oxygen atom has gained only one electron, not two. The oxygen atoms are bonded to each other, forming an O-O bond, which accounts for the unusual oxidation state. The O-O bond is relatively weak, contributing to the instability and reactivity of peroxides.
Superoxides: An Oxidation State of -1/2
Superoxides represent another exception. In superoxides, each oxygen atom has an oxidation state of -1/2. This indicates a more complex electron distribution than in typical oxides or peroxides. The presence of an unpaired electron leads to paramagnetic properties and increased reactivity.
These exceptions, however, do not negate the general rule that oxygen predominantly acts as an oxidizing agent, gaining electrons in chemical reactions.
The Significance of Oxygen's Electron Gain
Oxygen's strong tendency to gain electrons is crucial for several reasons:
-
Energy Production: The reduction of oxygen during respiration is fundamental to energy production in living organisms. The energy released from the oxidation of glucose is harnessed to produce ATP, the primary energy currency of cells.
-
Combustion and Industrial Processes: The ability of oxygen to oxidize fuels is vital for combustion processes, powering engines, generating electricity, and driving industrial reactions.
-
Environmental Processes: Oxygen plays a key role in various environmental processes, including weathering, the breakdown of organic matter, and the formation of various minerals.
-
Material Science: Oxygen's reactivity impacts material science, influencing the properties of many materials and affecting corrosion and degradation processes.
-
Medical Applications: Oxygen is essential for human life and is utilized in medical applications, including respiratory support and wound healing.
Conclusion: Oxygen's Predominant Role as an Oxidizing Agent
In summary, while there are exceptions, oxygen overwhelmingly gains electrons in chemical reactions. This electron gain, characteristic of its high electronegativity and electron configuration, is the foundation of its oxidizing power. This oxidizing ability is paramount in numerous natural and industrial processes, making oxygen a central element in many aspects of our world. Understanding oxygen's electron behavior is key to comprehending its vital role in chemistry, biology, and numerous other scientific fields. The interplay between oxygen's electron gain and the simultaneous electron loss by other substances drives a vast array of essential chemical processes, shaping everything from the energy that fuels our bodies to the reactions that shape our planet.
Latest Posts
Latest Posts
-
70 Is What Percent Of 80
Mar 30, 2025
-
How Many Feet Is 236 Inches
Mar 30, 2025
-
Least Common Multiple Of 10 And 5
Mar 30, 2025
-
Difference Between Chemical Change And Chemical Property
Mar 30, 2025
-
8 Pints Is How Many Cups
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Does Oxygen Lose Or Gain Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
