Elements That Can Conduct Electricity Are Called
Kalali
Mar 19, 2025 · 6 min read
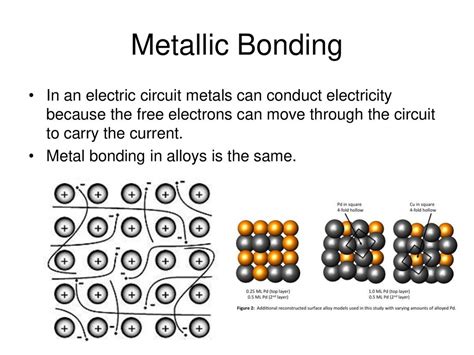
Table of Contents
Elements That Can Conduct Electricity Are Called Conductors: A Deep Dive
Elements that can conduct electricity are called conductors. This seemingly simple statement opens the door to a fascinating world of physics, chemistry, and engineering. Understanding conductivity is crucial for numerous applications, from the power grid lighting our homes to the intricate circuits in our smartphones. This comprehensive article will explore the fundamental principles behind electrical conductivity, delve into the specific properties of conductive elements, and discuss the various applications of these materials in modern technology.
What is Electrical Conductivity?
At the heart of electrical conductivity lies the ability of a material to allow the flow of electric charge. This flow, typically in the form of electrons, is driven by an applied electric field. Materials are classified based on their conductivity into three broad categories:
- Conductors: Materials that readily allow the flow of electric charge. These materials have a large number of free electrons that can move easily through the material.
- Insulators: Materials that strongly resist the flow of electric charge. These materials have tightly bound electrons, preventing their movement.
- Semiconductors: Materials with conductivity intermediate between conductors and insulators. Their conductivity can be manipulated by external factors like temperature or doping.
The ease with which electrons move through a material is quantified by its electrical conductivity, often represented by the Greek letter σ (sigma). The inverse of conductivity is resistivity (ρ, rho), which measures a material's resistance to the flow of current. Good conductors have high conductivity and low resistivity, while good insulators have low conductivity and high resistivity.
The Role of Atomic Structure
The ability of an element to conduct electricity is fundamentally linked to its atomic structure and, more specifically, its electron configuration. Conductors typically have loosely bound electrons in their outermost electron shells (valence electrons). These electrons are relatively free to move throughout the material's lattice structure when an electric field is applied.
Metals, the quintessential conductors, are characterized by their metallic bonding. In metallic bonding, valence electrons are delocalized, meaning they are not associated with any particular atom but rather form a "sea" of electrons that are free to move throughout the metal's structure. This "sea" of electrons is responsible for the high electrical conductivity of metals.
Factors Affecting Conductivity
Several factors can influence the electrical conductivity of a material:
- Temperature: In most conductors, conductivity decreases with increasing temperature. Higher temperatures increase the vibrational energy of the atoms in the lattice, hindering the movement of electrons.
- Impurities: The presence of impurities in a conductor can scatter electrons, reducing conductivity. This is why high-purity metals are generally preferred in electrical applications.
- Crystal Structure: The arrangement of atoms in a material's crystal structure can affect electron mobility. A well-ordered structure generally leads to higher conductivity.
- Pressure: Pressure can also affect conductivity, although the effect is often less significant than temperature or impurities.
Key Conductive Elements
While many materials exhibit electrical conductivity, certain elements stand out for their exceptional conductive properties. These elements are primarily found in the left-hand side of the periodic table, where the valence electrons are loosely bound.
Copper (Cu)
Copper is arguably the most widely used conductor in electrical applications. Its high conductivity, excellent ductility (ability to be drawn into wires), and relatively low cost make it ideal for wiring, cabling, and electrical components. The ubiquitous use of copper in electrical systems underscores its importance in modern infrastructure.
Silver (Ag)
Silver possesses even higher conductivity than copper. However, its significantly higher cost limits its use to specialized applications where superior conductivity is paramount, such as high-frequency circuits and specialized electrical contacts.
Gold (Au)
Gold is another excellent conductor, prized for its resistance to corrosion and oxidation. This makes it suitable for applications requiring long-term reliability and stability, such as connectors and surface mount devices in electronics. Its inertness is a significant advantage in many applications.
Aluminum (Al)
Aluminum offers a lighter and cheaper alternative to copper, although its conductivity is lower. This makes it suitable for overhead power lines, where weight is a critical factor. Aluminum is increasingly used in various electrical applications, driving down costs.
Other Conductive Elements
Other elements with significant conductivity include:
- Iron (Fe): While not as conductive as copper or silver, iron is widely used in electrical machinery and transformers due to its magnetic properties in addition to its reasonable conductivity.
- Tungsten (W): Possesses high melting point, making it suitable for filaments in incandescent light bulbs. While its conductivity is lower than that of copper, this property is crucial for its application.
- Mercury (Hg): A liquid metal with unique properties, mercury finds application in specialized switches and sensors. However, its toxicity limits its use.
Applications of Conductive Elements
The applications of conductive elements are vast and span diverse fields:
Power Generation and Transmission
Copper and aluminum are essential in power generation and transmission. Copper wires are commonly used in electrical systems to conduct electricity from power plants to homes and businesses, while aluminum is used for high-voltage transmission lines due to its light weight.
Electronics
Conductive elements are the backbone of electronic devices. Printed circuit boards (PCBs) use copper tracks to connect various components. In integrated circuits, gold and other conductive metals are employed to form interconnects and contacts. The miniaturization of electronics relies heavily on the precise control and manipulation of conductive materials.
Telecommunications
High-frequency applications in telecommunications necessitate materials with high conductivity and low skin effect. Silver and gold are frequently chosen for their superior performance in these applications.
Transportation
Electric vehicles rely on conductive materials for their power systems. Copper and aluminum are crucial components in electric motors, batteries, and charging infrastructure. The increasing adoption of electric vehicles further underscores the importance of conductive materials.
Medical Devices
Conductive materials are critical components in various medical devices, including pacemakers, defibrillators, and electrodiagnostic equipment. The biocompatibility and reliability of these materials are crucial for ensuring patient safety.
Heating Elements
Certain conductive elements exhibit high resistivity, allowing them to generate heat when current is passed through them. This property is utilized in heating elements for appliances like toasters and electric heaters. Materials like Nichrome (a nickel-chromium alloy) are commonly used due to their high resistivity and oxidation resistance.
Conclusion
Elements that can conduct electricity, primarily metals, are fundamental to our modern technological society. Their ability to facilitate the flow of electric charge underpins countless applications, from the ubiquitous power grid to the sophisticated circuitry of smartphones. Understanding the underlying principles of electrical conductivity, the properties of individual conductive elements, and the factors influencing their behavior is crucial for ongoing innovation and the development of new technologies. As research continues to explore new materials and their properties, the applications of conductive elements will continue to expand, shaping the future of technology and driving advancements across various industries. This intricate relationship between atomic structure, material properties, and technological applications underlines the fundamental importance of conductive elements in our daily lives. Further exploration into the nuances of conductivity, especially within the context of specific material combinations and emerging technologies, promises exciting discoveries and innovations.
Latest Posts
Latest Posts
-
How Many Grams Are In A Half Pound
Mar 19, 2025
-
Convert 35 C To Degrees Fahrenheit
Mar 19, 2025
-
How Many Cups Is Six Ounces
Mar 19, 2025
-
How Many Feet Is 140 Inches
Mar 19, 2025
-
How Many Feet Is 22 Meters
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Elements That Can Conduct Electricity Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
