Emission Of Light From An Atom Occurs When An Electron
Kalali
Mar 14, 2025 · 6 min read
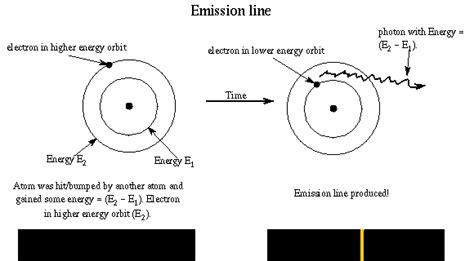
Table of Contents
Emission of Light from an Atom Occurs When an Electron…
The emission of light from an atom is a fundamental process in physics, crucial to understanding phenomena ranging from the colors of stars to the operation of lasers. This process, known as atomic emission, occurs when an electron within an atom transitions from a higher energy level to a lower energy level. Let's delve deeper into this fascinating phenomenon, exploring the underlying mechanisms, the different types of emission, and the applications that stem from this fundamental interaction.
The Bohr Model and Energy Levels
To understand atomic emission, we need to grasp the concept of electron energy levels. The simplest model, though admittedly a simplification, is the Bohr model. This model depicts electrons orbiting the nucleus at specific, discrete energy levels. These levels are quantized, meaning electrons can only exist at these specific energy levels and not in between. Each energy level is associated with a particular energy value. The lowest energy level is called the ground state, while higher energy levels are called excited states.
The key takeaway here is that electrons are not free to roam anywhere around the nucleus; they occupy specific, defined energy shells or orbitals. The energy of an electron is directly related to its distance from the nucleus: the farther away, the higher the energy.
Transitions Between Energy Levels
An atom typically exists in its ground state, with its electrons occupying the lowest available energy levels. However, energy can be added to the atom through various means, such as:
- Absorption of a photon: A photon of light with energy precisely matching the energy difference between two levels can be absorbed by an electron, causing it to jump to a higher energy level. This process is called absorption.
- Collision with another atom or particle: A collision with sufficient kinetic energy can also excite an electron to a higher energy level. This is common in high-temperature environments like stars and flames.
- Electrical discharge: Passing electricity through a gas can also provide the energy needed for electron excitation.
This excited state is unstable. The electron will naturally want to return to a lower energy level, a more stable state. This transition from a higher energy level to a lower energy level is accompanied by the emission of a photon. The energy of the emitted photon is exactly equal to the energy difference between the two levels.
The Nature of Emitted Light
The energy of the emitted photon directly determines the frequency and wavelength of the light. This relationship is described by Planck's equation:
E = hf = hc/λ
where:
- E is the energy of the photon
- h is Planck's constant
- f is the frequency of the light
- c is the speed of light
- λ is the wavelength of the light
Since the energy levels within an atom are quantized, the emitted light will have specific, discrete wavelengths. This results in a line spectrum, a characteristic pattern of bright lines at specific wavelengths, unique to each element. This is how we can identify the elements present in a sample using atomic emission spectroscopy.
Types of Atomic Emission
While the fundamental principle remains the same – an electron transitioning to a lower energy level emits a photon – there are various types of atomic emission, each with its own characteristics:
1. Spontaneous Emission
This is the most common type of emission. After an electron is excited to a higher energy level, it spontaneously transitions back to a lower energy level, emitting a photon without any external stimulus. The timing of this transition is random.
2. Stimulated Emission
This type of emission forms the basis of lasers. When an atom is in an excited state, a photon of the exact energy needed for the transition can stimulate the electron to fall back to a lower energy level, emitting a second photon identical to the stimulating photon. These two photons are in phase, coherent, and this process leads to amplification of light.
3. Fluorescence and Phosphorescence
These are related processes where a molecule or atom absorbs light and then re-emits light at a longer wavelength. The difference lies in the timescale of the emission: fluorescence occurs almost immediately after absorption, while phosphorescence can persist for a longer period. This difference is due to the involvement of different energy levels and transition pathways.
Applications of Atomic Emission
The emission of light from atoms has numerous applications across various scientific and technological fields:
-
Spectroscopy: Atomic emission spectroscopy (AES) is used to identify the elements present in a sample by analyzing the wavelengths of light emitted when the sample is excited. This technique is widely used in analytical chemistry, environmental monitoring, and material science.
-
Astronomy: The light emitted by stars and other celestial objects provides crucial information about their composition, temperature, and motion. Analyzing the spectral lines allows astronomers to determine the abundance of different elements in stars and galaxies.
-
Lighting: Many lighting technologies, such as sodium vapor lamps and neon lights, rely on the emission of light from excited atoms. These lamps are efficient and produce light of specific wavelengths, making them suitable for various applications.
-
Lasers: Lasers, which rely on stimulated emission, are used extensively in various fields, including medicine, telecommunications, and manufacturing. The high intensity and coherence of laser light make them incredibly versatile tools.
-
Medical Imaging: Certain medical imaging techniques, such as atomic absorption spectrometry, use atomic emission principles to analyze biological samples and diagnose various medical conditions.
Quantum Mechanics and a More Accurate Picture
While the Bohr model provides a basic understanding of atomic emission, a more accurate description requires the principles of quantum mechanics. Quantum mechanics reveals that electrons are not simply orbiting the nucleus but are described by wave functions that represent the probability of finding the electron at a particular location. These wave functions are associated with specific energy levels and orbitals, leading to the quantization of energy.
The precise calculation of energy levels and transition probabilities requires solving the Schrödinger equation for the atom in question. This is often a complex task, especially for multi-electron atoms. However, sophisticated computational methods allow for accurate predictions of atomic spectra.
Conclusion
The emission of light from an atom, driven by the transition of an electron between energy levels, is a fundamental process with profound implications across various fields. From the vibrant colors of fireworks to the precise measurements in analytical chemistry and the powerful beams of lasers, the understanding and application of this process have revolutionized our technology and our understanding of the universe. The journey from the simplistic Bohr model to the complexities of quantum mechanics highlights the ongoing evolution of our understanding of this fundamental phenomenon, and promises further discoveries and applications in the future. Further exploration into this topic involves delving into finer details of quantum electrodynamics, the study of the interaction between light and matter at a quantum level, offering an even more complete and accurate picture of this fascinating process. The continued research in this area is crucial to advancing our knowledge and technological capabilities.
Latest Posts
Latest Posts
-
What Is Ten Percent Of 100
Mar 14, 2025
-
How Many Feet Is 1000 Meters
Mar 14, 2025
-
How Many Feet Are In 108 Inches
Mar 14, 2025
-
How Many Ounces Is 75 Ml
Mar 14, 2025
-
How Much Is 8 Ounces Water
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Emission Of Light From An Atom Occurs When An Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
