Example Of A Gas-liquid Solute Solvent Combination
Kalali
Mar 24, 2025 · 6 min read
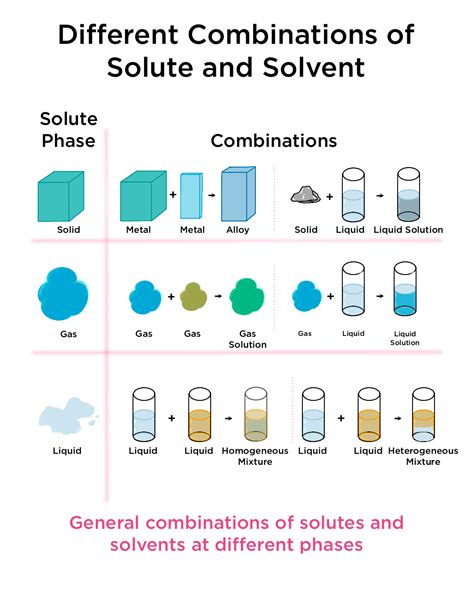
Table of Contents
Examples of Gas-Liquid Solute-Solvent Combinations: A Deep Dive
Understanding gas-liquid solute-solvent combinations is crucial in various fields, from chemical engineering and environmental science to medicine and food technology. This comprehensive guide delves into numerous examples, exploring the principles behind solubility, influencing factors, and practical applications. We'll explore both natural and engineered systems, highlighting the diverse ways gases dissolve in liquids and the implications of these interactions.
Defining the Terms: Solute, Solvent, and Solution
Before diving into specific examples, let's establish a clear understanding of the key terms:
-
Solute: This is the substance that dissolves in a solvent. In gas-liquid systems, the solute is a gas. Examples include carbon dioxide, oxygen, nitrogen, and sulfur dioxide.
-
Solvent: This is the substance that dissolves the solute. In our context, the solvent is a liquid. Common examples include water, ethanol, organic solvents like hexane, and specialized solvents used in industrial processes.
-
Solution: The homogeneous mixture formed when a solute dissolves in a solvent. The gas is uniformly dispersed throughout the liquid at the molecular level.
Factors Affecting Gas Solubility in Liquids
Several factors significantly influence the solubility of a gas in a liquid:
-
Temperature: Generally, gas solubility decreases with increasing temperature. Higher temperatures provide gas molecules with greater kinetic energy, allowing them to escape the liquid phase.
-
Pressure: According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid. Increasing the pressure increases the gas solubility.
-
Nature of the Gas and Solvent: The chemical nature of both the gas and the liquid plays a crucial role. Polar gases tend to dissolve better in polar solvents (like water), while nonpolar gases dissolve better in nonpolar solvents (like hexane). This is based on the principle of "like dissolves like."
-
Presence of Other Solutes: The presence of other solutes in the liquid can affect the solubility of a gas. Ionic compounds or other gases can compete for space and interactions with the solvent molecules, influencing the overall gas solubility.
Examples of Gas-Liquid Solute-Solvent Combinations
Now, let's explore a diverse range of examples, categorizing them for clarity:
1. Carbon Dioxide in Water (Carbonated Beverages)
This is perhaps the most ubiquitous example. Carbon dioxide gas is dissolved under pressure in water to create carbonated drinks. The fizzing sensation results from the release of CO2 when the pressure is reduced. The solubility of CO2 in water is relatively low, but sufficient pressure ensures a significant amount dissolves, contributing to the characteristic taste and effervescence. Temperature plays a crucial role; warm sodas go flat faster because CO2 solubility decreases with increasing temperature.
2. Oxygen in Blood (Respiration)
Oxygen is crucial for respiration in most living organisms. In humans and other mammals, oxygen dissolves in the blood, primarily bound to hemoglobin in red blood cells. The solubility of oxygen in blood plasma is relatively low, which is why hemoglobin's oxygen-carrying capacity is essential. Factors like altitude (affecting partial pressure of oxygen) and temperature influence oxygen solubility and therefore oxygen uptake by the blood.
3. Nitrogen in Blood (Diving and Decompression Sickness)
At elevated pressures, like those encountered during scuba diving, nitrogen's solubility in blood increases significantly. During ascent, the pressure decreases, and dissolved nitrogen forms bubbles in the blood and tissues. This can lead to decompression sickness ("the bends"), a serious condition requiring careful decompression protocols. This example highlights the dangers of ignoring the relationship between pressure and gas solubility.
4. Sulfur Dioxide in Water (Acid Rain)
Sulfur dioxide (SO2), a major air pollutant, dissolves readily in rainwater, forming sulfurous acid (H2SO3) and contributing to acid rain. The solubility of SO2 in water is relatively high, making it a potent contributor to environmental acidification. Acid rain has detrimental effects on ecosystems, damaging forests, aquatic life, and infrastructure. This illustrates the environmental significance of gas-liquid interactions.
5. Ammonia in Water (Household Cleaners)
Ammonia gas (NH3) is highly soluble in water. Household ammonia cleaners are dilute solutions of ammonia in water. The solution is basic, due to ammonia's ability to accept protons from water. The solubility of ammonia is temperature-dependent, and concentrations must be carefully controlled for safety, given ammonia's toxicity.
6. Chlorine in Water (Water Purification)
Chlorine gas (Cl2) is used to disinfect water supplies. It dissolves in water to form hypochlorous acid (HOCl) and hypochlorite ions (OCl-), which are powerful disinfectants. The solubility of chlorine in water is moderate, and the effectiveness of water disinfection depends on chlorine concentration and contact time.
7. Hydrogen Sulfide in Water (Oil and Gas Industry)
Hydrogen sulfide (H2S), a toxic and flammable gas, is often found in natural gas and crude oil. Its solubility in water is influenced by pressure and temperature. In oil and gas operations, managing H2S solubility is crucial for safety and environmental reasons. Specialized techniques are employed to remove H2S from natural gas before distribution.
8. Noble Gases in Water (Environmental Monitoring)
Noble gases like helium, neon, argon, krypton, xenon, and radon have very low solubility in water. However, their presence and concentrations in water can be used as tracers in environmental studies and groundwater monitoring. The analysis of noble gas concentrations can provide insights into water sources, flow patterns, and potential contamination.
9. Volatile Organic Compounds (VOCs) in Water (Pollution Control)
Many volatile organic compounds (VOCs), such as benzene, toluene, and trichloroethylene, are relatively insoluble in water but can still contaminate water sources. Their partitioning between the air and water phases is governed by Henry's Law and is a key aspect of environmental remediation strategies. Understanding their solubility behavior is critical in developing effective water treatment methods.
10. Pharmaceutical Gases in Liquid Formulations (Drug Delivery)
Some pharmaceuticals exist as gases (e.g., certain anesthetics) but are dissolved in liquid carriers for delivery. The solubility of these gases in the chosen solvent is a crucial factor in ensuring effective drug delivery and controlling the release rate. The stability and effectiveness of the drug depend on the careful selection of both the gas and liquid.
Applications and Significance
Gas-liquid solute-solvent combinations have widespread applications in numerous fields:
-
Food and Beverage Industry: Carbonated drinks, beer brewing, and food preservation are all significantly influenced by gas solubility.
-
Chemical Engineering: Gas absorption, distillation, and extraction processes rely heavily on understanding and manipulating gas solubility.
-
Environmental Science: Understanding gas solubility is critical for assessing air and water pollution, modeling atmospheric processes, and designing remediation strategies.
-
Medicine and Pharmacy: Gas solubility plays a critical role in drug delivery, respiratory therapy, and understanding physiological processes.
-
Oceanography and Meteorology: Gas solubility is a key factor in ocean-atmosphere interactions and climate modeling.
Conclusion
The diverse examples explored above demonstrate the pervasive importance of gas-liquid solute-solvent combinations. From the simple act of enjoying a carbonated drink to the complexities of environmental monitoring and drug delivery, understanding the factors influencing gas solubility is essential. This knowledge allows for the design and optimization of processes across various industries and contributes to a deeper understanding of natural phenomena. Further research and development in this area will undoubtedly lead to even more innovative applications in the future.
Latest Posts
Latest Posts
-
How Many Cm Is 2 Meters
Mar 28, 2025
-
How Many Liters Is 1500 Milliliters
Mar 28, 2025
-
What Percentage Is 17 Out Of 20
Mar 28, 2025
-
100 Degrees Is What In Celsius
Mar 28, 2025
-
What Is The Percentage Of 5 Out Of 6
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Example Of A Gas-liquid Solute Solvent Combination . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
