Gaseous Butane Will React With Gaseous Oxygen
Kalali
Apr 03, 2025 · 5 min read
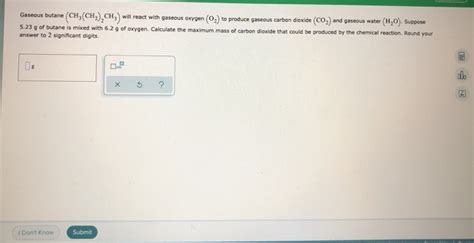
Table of Contents
- Gaseous Butane Will React With Gaseous Oxygen
- Table of Contents
- Gaseous Butane Reacting with Gaseous Oxygen: A Deep Dive into Combustion
- Understanding the Stoichiometry: The Balanced Equation
- Incomplete Combustion: A Less Efficient Process
- Thermodynamics of Butane Combustion: Energy Release
- Calculating Heat Released: Practical Applications
- Reaction Kinetics and Activation Energy
- Factors Influencing Reaction Rate
- Real-World Applications and Safety Considerations
- Safety Concerns Associated with Butane Combustion
- Environmental Impact: A Consideration for Sustainable Practices
- Conclusion: Balancing Utility with Responsibility
- Latest Posts
- Related Post
Gaseous Butane Reacting with Gaseous Oxygen: A Deep Dive into Combustion
The reaction between gaseous butane (C₄H₁₀) and gaseous oxygen (O₂) is a classic example of a combustion reaction, specifically a hydrocarbon combustion. This process, often utilized for heating and cooking, releases a significant amount of energy in the form of heat and light. Understanding this reaction, from its stoichiometry to its real-world applications and safety considerations, is crucial for various fields, including chemistry, engineering, and safety management.
Understanding the Stoichiometry: The Balanced Equation
The complete combustion of butane involves the reaction of butane with oxygen to produce carbon dioxide (CO₂) and water (H₂O). The balanced chemical equation for this reaction is:
2C₄H₁₀(g) + 13O₂(g) → 8CO₂(g) + 10H₂O(g)
This equation signifies that two moles of butane react with thirteen moles of oxygen to produce eight moles of carbon dioxide and ten moles of water. The (g) notation indicates that all reactants and products are in the gaseous phase under typical combustion conditions. It's crucial to understand this stoichiometry to calculate the amounts of reactants needed or products formed in a given reaction. For instance, knowing the mass of butane burned allows us to calculate the mass of carbon dioxide produced, a critical calculation for understanding the environmental impact of butane combustion.
Incomplete Combustion: A Less Efficient Process
While the equation above represents complete combustion, under conditions with insufficient oxygen, incomplete combustion occurs. This results in the formation of carbon monoxide (CO) and/or soot (carbon, C), along with carbon dioxide and water. The equations for incomplete combustion are more complex and varied, depending on the oxygen availability. Examples include:
- 2C₄H₁₀(g) + 9O₂(g) → 8CO(g) + 10H₂O(g) (Producing carbon monoxide)
- 2C₄H₁₀(g) + 7O₂(g) → 8C(s) + 10H₂O(g) (Producing soot)
Incomplete combustion is less efficient in terms of energy released and is also significantly more dangerous due to the production of toxic carbon monoxide, a colorless and odorless gas that can be fatal. The presence of soot can also contribute to air pollution and respiratory problems. Ensuring sufficient oxygen supply is therefore paramount in any butane-burning application.
Thermodynamics of Butane Combustion: Energy Release
The combustion of butane is an exothermic reaction, meaning it releases heat. The amount of heat released can be calculated using the enthalpy of combustion (ΔH<sub>c</sub>). The enthalpy of combustion of butane is approximately -2877 kJ/mol. This negative value indicates that the reaction releases energy, making it a valuable fuel source.
Calculating Heat Released: Practical Applications
This enthalpy value allows us to calculate the amount of heat released during the burning of a specific mass or volume of butane. For example, knowing the molar mass of butane (58.12 g/mol), one can determine the heat released per gram of butane burned. Such calculations are essential in designing and optimizing combustion engines, heating systems, and other applications that utilize butane as a fuel. This precise calculation is also crucial in industrial safety assessments to predict the heat output and potential risks associated with large-scale butane usage.
Reaction Kinetics and Activation Energy
The rate at which butane reacts with oxygen is governed by reaction kinetics. Like all chemical reactions, butane combustion requires an initial input of energy, known as the activation energy, to initiate the reaction. This activation energy can be provided by a spark or a flame. Once the reaction starts, the heat released sustains the process, creating a self-sustaining combustion.
Factors Influencing Reaction Rate
Several factors influence the rate of butane combustion:
- Temperature: Higher temperatures increase the kinetic energy of the reactant molecules, leading to more frequent and energetic collisions, thus accelerating the reaction rate.
- Concentration of Reactants: A higher concentration of butane and oxygen leads to more frequent collisions between the reactant molecules, resulting in a faster reaction.
- Surface Area: In applications where butane is in contact with a catalyst or a large surface area, the reaction rate can be significantly enhanced.
- Presence of Catalysts: Catalysts can lower the activation energy, accelerating the reaction rate without being consumed in the process.
Real-World Applications and Safety Considerations
Butane's combustion reaction finds widespread applications:
- Heating: Butane is commonly used in portable camping stoves, butane gas cylinders for home heating, and some types of lighters.
- Cooking: Butane-powered stoves are prevalent in areas lacking access to other fuels.
- Industrial Processes: Certain industrial applications utilize butane combustion for specialized heating and power generation.
Safety Concerns Associated with Butane Combustion
The exothermic nature of butane combustion necessitates strict safety precautions:
- Fire Hazards: Butane is highly flammable; hence, storage and handling must adhere to safety regulations to prevent fires or explosions.
- Oxygen Depletion: Incomplete combustion can lead to oxygen depletion in enclosed spaces, causing suffocation.
- Carbon Monoxide Poisoning: Incomplete combustion produces toxic carbon monoxide, requiring proper ventilation in any application utilizing butane combustion.
- Pressure Build-up: The combustion process produces gaseous products. In confined spaces, pressure build-up can occur, leading to potential explosions if not properly managed.
Environmental Impact: A Consideration for Sustainable Practices
The combustion of butane contributes to greenhouse gas emissions, primarily carbon dioxide. While a vital fuel source, understanding its environmental impact is crucial for promoting sustainable practices. Minimizing incomplete combustion by ensuring sufficient oxygen supply and employing energy-efficient technologies are essential steps towards mitigating environmental consequences. Furthermore, exploring alternative and cleaner energy sources to gradually replace butane in various applications is vital for long-term environmental sustainability. Research into efficient carbon capture and storage technologies could also play a crucial role in mitigating the effects of butane combustion on the environment.
Conclusion: Balancing Utility with Responsibility
The reaction of gaseous butane with gaseous oxygen is a fundamental chemical process with widespread applications. Its exothermic nature and relatively easy accessibility make it a valuable fuel source. However, understanding the stoichiometry, thermodynamics, kinetics, and safety considerations is essential for responsible and efficient utilization. Minimizing environmental impact through complete combustion, efficient technology, and exploring alternative energy sources are vital steps towards sustainable practices. The knowledge and careful application of this scientific understanding allow us to harness the power of this reaction while minimizing potential hazards and environmental repercussions. Ongoing research and technological advancements continually refine our understanding and management of this crucial chemical process.
Latest Posts
Related Post
Thank you for visiting our website which covers about Gaseous Butane Will React With Gaseous Oxygen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.