Has 5 Valence Electrons And Is In Period 3
Kalali
Mar 21, 2025 · 6 min read
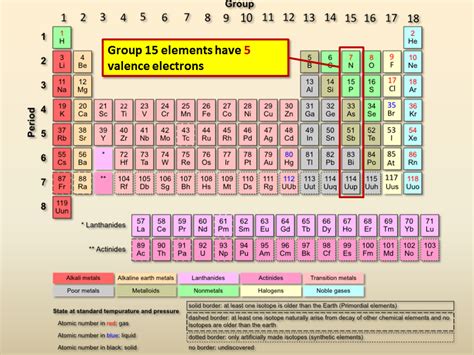
Table of Contents
Unveiling the Element with 5 Valence Electrons in Period 3: Phosphorus
Phosphorus, a nonmetal residing in period 3 and group 15 of the periodic table, is a fascinating element characterized by its five valence electrons. This unique electronic configuration dictates its diverse chemical behavior, leading to a myriad of applications across various fields, from fertilizers and detergents to biological systems and semiconductors. This article will delve deep into the properties, reactions, and significance of phosphorus, exploring its crucial role in our world.
Understanding Valence Electrons and Period 3
Before diving into the specifics of phosphorus, let's establish a foundational understanding of valence electrons and their significance in determining an element's properties. Valence electrons are the electrons located in the outermost shell of an atom, and they are the key players in chemical bonding. These electrons are responsible for the element's reactivity and its ability to form chemical bonds with other atoms.
Period 3 of the periodic table represents elements with three electron shells. This means that their outermost electrons reside in the third shell, influencing their properties and how they interact with other elements. The number of valence electrons increases across period 3, from sodium's single valence electron to argon's eight (a complete octet).
Phosphorus, with its five valence electrons, occupies a unique position in period 3. This number isn't a full octet, which drives its chemical reactivity and its tendency to form multiple bonds.
The Properties of Phosphorus: A Detailed Look
Phosphorus exists in several allotropic forms, meaning it can exist in different structural modifications with distinct physical and chemical properties. The most common forms are white phosphorus and red phosphorus.
White Phosphorus: Highly Reactive
White phosphorus (P₄) is a highly reactive, waxy, and translucent solid. It's extremely toxic and ignites spontaneously in air, producing a bright white flame and dense clouds of phosphorus pentoxide (P₄O₁₀). This remarkable reactivity stems directly from its five valence electrons and the strain within the P₄ tetrahedral molecule. Each phosphorus atom shares three electrons with three other phosphorus atoms, resulting in an unstable and highly reactive molecule.
Key Properties of White Phosphorus:
- Highly reactive: It readily reacts with oxygen, water, and halogens.
- Toxic: Exposure can lead to severe health consequences.
- Low melting point: Relatively low melting point compared to other allotropes.
- Luminescent: Emits a faint glow in the dark due to slow oxidation.
- Flammable: Ignites spontaneously in air.
Red Phosphorus: Less Reactive
Red phosphorus is a less reactive allotrope, appearing as a reddish-brown powder. It is significantly less toxic and does not ignite spontaneously in air. Its structure is amorphous, consisting of a complex network of phosphorus atoms linked together. This polymeric structure stabilizes the phosphorus atoms, reducing its reactivity compared to white phosphorus.
Key Properties of Red Phosphorus:
- Less reactive: More stable and less toxic than white phosphorus.
- Non-flammable (in air): Does not ignite spontaneously.
- Insoluble in water: Unlike white phosphorus, which is slightly soluble.
- Used in matches: A key component in the striking surface of safety matches.
Other Allotropes: Black and Violet Phosphorus
Beyond white and red phosphorus, there are other less common allotropes, such as black phosphorus and violet phosphorus. Black phosphorus is a layered material with metallic conductivity, while violet phosphorus possesses a more ordered crystalline structure than red phosphorus.
Chemical Reactions of Phosphorus: A Versatile Element
Phosphorus's five valence electrons make it a highly versatile element capable of forming a wide range of compounds. Its reactions primarily involve gaining three electrons to achieve a stable octet, or sharing electrons to form covalent bonds.
Reactions with Oxygen: Formation of Oxides
Phosphorus readily reacts with oxygen, forming various oxides, the most common being phosphorus pentoxide (P₄O₁₀). This reaction is highly exothermic, releasing significant heat. The formation of P₄O₁₀ demonstrates phosphorus's tendency to achieve an octet configuration by forming multiple bonds with oxygen.
Reactions with Halogens: Formation of Halides
Phosphorus reacts vigorously with halogens (fluorine, chlorine, bromine, and iodine) to form various phosphorus halides. For example, the reaction with chlorine produces phosphorus trichloride (PCl₃) and phosphorus pentachloride (PCl₅). These reactions showcase phosphorus's ability to form both three and five covalent bonds depending on the halogen's reactivity.
Reactions with Metals: Formation of Phosphides
Phosphorus reacts with many metals to form metal phosphides. These compounds typically have ionic character, with phosphorus present as phosphide anions (P³⁻). The formation of phosphides illustrates phosphorus's ability to gain electrons to achieve a stable octet.
Reactions with Acids: Formation of Phosphoric Acid
The reaction of phosphorus with concentrated nitric acid leads to the formation of phosphoric acid (H₃PO₄). This reaction is an example of an oxidation reaction, where phosphorus is oxidized to its highest oxidation state (+5). Phosphoric acid is a vital compound with extensive applications.
The Importance of Phosphorus in Biological Systems
Phosphorus plays a crucial role in all living organisms. It is an essential component of:
- Nucleic acids (DNA and RNA): The backbone of DNA and RNA molecules contains phosphate groups linking the sugar molecules together. These molecules are vital for storing and transferring genetic information.
- ATP (Adenosine Triphosphate): ATP is the primary energy currency of cells. The energy released during ATP hydrolysis is used to power various cellular processes.
- Phospholipids: Phospholipids are major components of cell membranes, forming a bilayer that separates the cell's internal environment from its surroundings.
- Bones and teeth: Phosphorus is a vital constituent of bones and teeth, contributing to their strength and structure.
Applications of Phosphorus: A Broad Spectrum
The unique properties and chemical reactivity of phosphorus have led to its widespread use in various applications:
- Fertilizers: Phosphorus is an essential nutrient for plant growth and is a major component of many fertilizers. Phosphates are crucial for root development, flowering, and fruiting.
- Detergents: Phosphates were commonly used in detergents as builders to soften water, however, their use has been restricted due to environmental concerns regarding eutrophication.
- Matches: Red phosphorus is a key component in the striking surface of safety matches.
- Semiconductors: Phosphorus is used in the semiconductor industry as a dopant in silicon to create n-type semiconductors.
- Flame retardants: Organophosphorus compounds are used as flame retardants in various materials, such as plastics and textiles.
- Pesticides: Certain organophosphorus compounds are used as insecticides and herbicides.
Environmental Considerations: Phosphorus Pollution
While phosphorus is essential for life, excessive amounts can lead to environmental problems. Runoff from agricultural lands containing phosphorus-based fertilizers contributes to eutrophication in water bodies. This process involves the excessive growth of algae, leading to oxygen depletion and harming aquatic life. Therefore, responsible use and management of phosphorus are critical for environmental sustainability.
Conclusion: An Essential Element with Diverse Roles
Phosphorus, with its five valence electrons in period 3, is a pivotal element with a vast array of applications and vital roles in biological systems. Its unique chemical properties and reactivity have made it indispensable in agriculture, industry, and our understanding of life itself. However, responsible management of phosphorus is crucial to mitigate its potential environmental impacts and ensure its sustainable use for future generations. Continued research into its various allotropes, chemical reactions, and biological functions will undoubtedly reveal even more about this fascinating and essential element. Understanding its reactivity, its various allotropes, and its crucial role in biological systems underscores the importance of phosphorus in our world. Careful consideration of its environmental impact will ensure its continued benefits for society while safeguarding our planet's health.
Latest Posts
Latest Posts
-
9 Is What Percent Of 12
Mar 28, 2025
-
Cuanto Son 100 Pies A Metros
Mar 28, 2025
-
How Many Ounces Of Sour Cream Are In A Cup
Mar 28, 2025
-
Does A Catfish Have A Backbone
Mar 28, 2025
-
How Many Feet Are In 42 Inches
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Has 5 Valence Electrons And Is In Period 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
