Horizontal Row In The Periodic Table Is Called
Kalali
Mar 10, 2025 · 7 min read
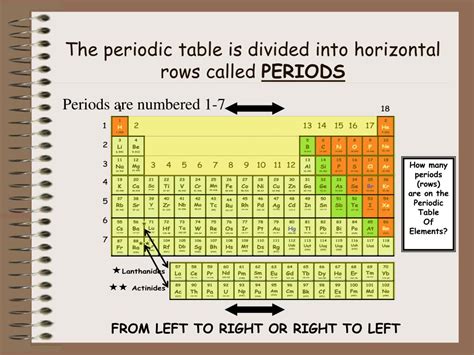
Table of Contents
A Horizontal Row in the Periodic Table is Called a Period: Understanding the Organization of Elements
The periodic table, a cornerstone of chemistry, organizes the chemical elements in a structured manner, revealing patterns and relationships in their properties. Understanding this organization is fundamental to grasping chemical behavior and predicting the characteristics of different elements. A key aspect of this organization involves the horizontal rows, which are known as periods. This article delves deep into the concept of periods in the periodic table, explaining their significance, how they relate to electron configurations, and their influence on elemental properties.
What is a Period in the Periodic Table?
A period in the periodic table is a horizontal row of chemical elements. These rows are numbered from 1 to 7, representing the principal energy levels or electron shells within an atom. Each element within a period shares the same highest principal quantum number (n), which defines the energy level of its outermost electrons (valence electrons). This commonality in the principal quantum number directly affects the chemical properties of the elements within that period.
The Significance of Principal Quantum Number (n)
The principal quantum number (n) is a crucial concept in understanding the structure of atoms. It dictates the energy level of an electron and, indirectly, the size of the electron shell. As we move across a period from left to right, electrons are added to the same principal energy level. This filling of the outermost electron shell progressively influences the atomic radius, ionization energy, and electronegativity of the elements.
How Periods Relate to Electron Configuration
The arrangement of electrons in an atom's electron shells is called its electron configuration. This configuration is intimately linked to the position of an element in the periodic table, specifically its period. Each period corresponds to the filling of a particular principal energy level.
- Period 1: Contains only hydrogen (H) and helium (He). Both have electrons in the first principal energy level (n=1), which can hold a maximum of two electrons.
- Period 2: Elements in period 2 have electrons filling the second principal energy level (n=2), which can accommodate up to eight electrons. This period includes elements like lithium (Li), beryllium (Be), boron (B), carbon (C), nitrogen (N), oxygen (O), fluorine (F), and neon (Ne).
- Period 3: Similar to period 2, period 3 elements fill the third principal energy level (n=3), also holding up to eight electrons. This period contains sodium (Na), magnesium (Mg), aluminum (Al), silicon (Si), phosphorus (P), sulfur (S), chlorine (Cl), and argon (Ar).
- Periods 4-7: The pattern continues for periods 4-7, each filling successively higher principal energy levels, although the number of electrons accommodated becomes more complex due to the introduction of d and f orbitals (which we'll discuss later).
Subshells and Orbitals: A Deeper Dive
Each principal energy level (n) is further divided into subshells (s, p, d, f), each capable of holding a specific number of electrons. These subshells are filled in a predictable order, following the Aufbau principle (filling from lowest to highest energy). This order is not strictly numerical but follows a specific sequence related to the energy levels of each subshell.
- s subshell: Can hold a maximum of 2 electrons.
- p subshell: Can hold a maximum of 6 electrons.
- d subshell: Can hold a maximum of 10 electrons.
- f subshell: Can hold a maximum of 14 electrons.
The filling of these subshells explains the varying lengths of the periods. Periods 1 and 2 are short because only the s and p subshells of the first two principal energy levels are being filled. Periods 3 and 4 are longer due to the additional electrons filling the 3d and 4d orbitals respectively. Periods 6 and 7 are the longest, incorporating the filling of the 4f and 5f orbitals, respectively – resulting in the lanthanides and actinides series.
Trends in Properties Across a Period
The elements within a period exhibit a predictable trend in their properties as we move from left to right. These trends are primarily driven by the increasing nuclear charge and the addition of electrons to the same principal energy level.
Atomic Radius
The atomic radius generally decreases across a period. This is because the increasing nuclear charge attracts the outer electrons more strongly, pulling them closer to the nucleus.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, generally increases across a period. The stronger nuclear pull makes it increasingly difficult to remove an electron.
Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across a period. This is a direct consequence of the increasing nuclear charge and the decrease in atomic radius.
Metallic Character
Metallic character, which encompasses properties such as malleability, ductility, and conductivity, generally decreases across a period. As we move from left to right, elements become less metallic and more non-metallic, culminating in the noble gases (group 18) exhibiting extremely low reactivity.
The Importance of Periods in Chemical Understanding
Understanding periods in the periodic table is crucial for several reasons:
- Predicting Properties: The periodic table's organization allows us to predict the properties of an element based on its position. Knowing an element's period gives insights into its electron configuration, reactivity, and chemical behavior.
- Understanding Chemical Reactions: The trends across periods help explain why certain elements react in specific ways. For instance, the high electronegativity of elements on the right side of a period explains their tendency to form anions (negatively charged ions).
- Developing New Materials: The periodic table is an invaluable tool in the development of new materials with desired properties. Understanding the trends across periods enables scientists to design materials with tailored characteristics.
- Categorizing Elements: Periods help categorize elements into groups with similar properties, facilitating the study and understanding of chemical processes.
Periods and the Structure of the Periodic Table
The structure of the periodic table itself is a reflection of the periodic trends observed in the elements’ properties. The periods are arranged horizontally, reflecting the filling of electron shells. The vertical columns, called groups, represent elements with similar valence electron configurations, leading to similar chemical behaviors.
The arrangement is not simply linear; it incorporates the filling of subshells (s, p, d, f) which leads to variations in period length and the presence of transition metals (d-block) and inner transition metals (f-block). These blocks represent the filling of different subshells and influence the properties of the elements significantly.
Advanced Concepts and Exceptions
While the trends discussed above generally hold true, exceptions do exist. These exceptions are often due to subtle variations in electron-electron repulsions, shielding effects, and the complexities of electron configurations in heavier elements.
For instance, some irregularities can be observed in ionization energy and electronegativity trends due to factors such as electron pairing energy and inter-electronic repulsion within subshells. These exceptions, however, do not invalidate the overall structure and utility of the periodic table.
Conclusion
The horizontal rows in the periodic table, known as periods, are fundamental to understanding the organization and properties of chemical elements. The periods directly correspond to the filling of principal energy levels within atoms, leading to predictable trends in atomic radius, ionization energy, electronegativity, and metallic character across each row. This structured organization provides a powerful tool for predicting and understanding chemical behavior, facilitating the development of new materials, and deepening our comprehension of the fundamental principles of chemistry. The periodic table's elegance lies in its ability to encompass the complex interplay of subatomic particles, leading to the diversity and predictable behavior of the elements around us. Understanding periods is therefore paramount for anyone seeking a comprehensive grasp of chemistry.
Latest Posts
Latest Posts
-
How Many Days In A Million Minutes
Jul 14, 2025
-
How Many Days Is In 11 Weeks
Jul 14, 2025
-
How Many Grams Are In One Tola Gold
Jul 14, 2025
-
How Many Oz In A Pound Of Freon
Jul 14, 2025
-
How Many Years Are In A Millennia
Jul 14, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Row In The Periodic Table Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.