How Are Elements And Compounds Alike
Kalali
Mar 14, 2025 · 6 min read
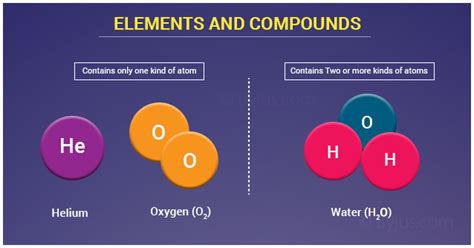
Table of Contents
How Are Elements and Compounds Alike? Exploring Similarities in the Building Blocks of Matter
Chemistry, at its core, is the study of matter and its transformations. Understanding the fundamental building blocks of matter – elements and compounds – is crucial to grasping the complexities of chemical reactions and the world around us. While seemingly distinct, elements and compounds share several fundamental similarities. This article delves deep into these similarities, exploring their shared characteristics at the atomic and molecular levels, their roles in forming mixtures, and their overall contribution to the diverse nature of matter.
Shared Characteristics at the Atomic Level
At the most basic level, both elements and compounds are composed of atoms. This is the most fundamental similarity, a cornerstone upon which all further comparisons are built. Atoms, the smallest units of matter that retain the chemical properties of an element, are the indivisible building blocks of both elements and compounds. They are made up of protons, neutrons, and electrons. The number of protons defines the element's identity, while the arrangement of electrons determines its chemical behavior.
Isotopes: A Shared Feature
Elements and compounds can both exist as isotopes. Isotopes are atoms of the same element (same number of protons) but with differing numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly. For instance, carbon-12 and carbon-14 are isotopes of carbon, both exhibiting similar chemical reactivities but differing in mass due to the extra neutrons in carbon-14. Similarly, compounds can also exhibit isotopic variations depending on the isotopic composition of the constituent elements.
Interaction through Chemical Bonds: The Unifying Force
Despite their differences in structure, both elements and compounds are held together, or interact, through various types of chemical bonds. These bonds, fundamentally electromagnetic interactions, involve the sharing or transfer of electrons between atoms. In elements, the atoms are of the same type, interacting through metallic bonds (as in metals) or weaker van der Waals forces (as in noble gases). In compounds, atoms of different elements interact through ionic bonds (involving electron transfer) or covalent bonds (involving electron sharing). The strength and nature of these bonds directly influence the properties of both elements and compounds.
Physical States: A Common Ground
Elements and compounds can exist in all three common states of matter: solid, liquid, and gas. The state they exist in depends on factors like temperature and pressure, which influence the kinetic energy of the atoms or molecules. For instance, oxygen exists as a gas at room temperature and pressure, while mercury is a liquid. Similarly, compounds like water can exist in all three states, transitioning between them based on changes in temperature and pressure. This demonstrates a fundamental similarity: their susceptibility to the same physical laws governing the states of matter.
Building Blocks of Mixtures: A Shared Role
Elements and compounds form the basic building blocks of mixtures. Mixtures are physically combined substances that retain their individual chemical identities. They can be homogeneous (uniform composition, like saltwater) or heterogeneous (non-uniform composition, like sand and water). The crucial aspect here is that neither elements nor compounds undergo chemical changes when forming a mixture. They can be separated through physical means like filtration or distillation. This demonstrates their ability to coexist without fundamentally altering their chemical structure, a shared attribute.
Examples of Elements and Compounds in Mixtures
Consider air, a homogeneous mixture. Air is primarily composed of the elements nitrogen and oxygen, along with smaller amounts of other gases like argon (another element) and carbon dioxide (a compound). Similarly, seawater is a homogeneous mixture containing the elements sodium and chlorine (which form the compound sodium chloride, or table salt) along with other dissolved elements and compounds. This illustrates how both elements and compounds are integral constituents of the natural world's numerous mixtures.
Chemical Reactions: Similar Participation
Both elements and compounds actively participate in chemical reactions. Chemical reactions involve the rearrangement of atoms and the breaking and formation of chemical bonds. While elements can react with each other to form compounds, compounds can also react with elements or other compounds to create new substances. This common capacity for change, for participating in chemical processes, highlights their fundamental interconnectedness within the broader context of chemical transformation.
Examples of Reactions Involving Elements and Compounds
Consider the combustion of methane (a compound). Methane reacts with oxygen (an element) to produce carbon dioxide (a compound) and water (another compound). This simple reaction highlights how both elements and compounds are indispensable actors in chemical processes, demonstrating their shared role in the dynamic world of chemical interactions.
The synthesis of water from hydrogen (an element) and oxygen (an element) is another illustrative example. Two elements combine chemically to form a compound. This exemplifies the capacity of elements to actively participate in the formation of compounds. Conversely, compounds can decompose into simpler elements or other compounds through reactions such as electrolysis or thermal decomposition. This highlights the dynamism and interconnectedness of elements and compounds within chemical processes.
The Role of Conservation Laws
Both elements and compounds adhere to the fundamental laws of conservation of mass and conservation of energy. The law of conservation of mass dictates that matter cannot be created or destroyed in a chemical reaction. The total mass of reactants equals the total mass of products. This principle applies equally to reactions involving elements and compounds. Similarly, the law of conservation of energy states that energy cannot be created or destroyed, only transformed. This applies universally, irrespective of whether the reaction involves elements or compounds. The adherence to these fundamental laws emphasizes the common underlying principles governing the behavior of both elements and compounds.
Analyzing Properties: Similarities and Differences
While both elements and compounds share the foundational aspects discussed above, their properties can differ significantly. Elements, by definition, possess unique chemical properties that are determined by their atomic number (number of protons). Their properties are intrinsic to the atom itself. Compounds, on the other hand, possess properties that are determined by the type of elements that compose them and the way those elements are bound together. A compound's properties are often very different from the properties of its constituent elements. For instance, sodium (a highly reactive metal) and chlorine (a toxic gas) combine to form sodium chloride (table salt), a relatively inert and edible substance.
However, both elements and compounds can be analyzed and characterized using various techniques. These include spectroscopic methods (like NMR and IR spectroscopy), chromatography, and various forms of microscopy. These techniques provide valuable information about their composition, structure, and properties, irrespective of whether the sample is an element or a compound. The shared applicability of these analytical tools underlines the common ground in how we study these fundamental building blocks of matter.
Conclusion: Unity in Diversity
Despite the distinct differences in their chemical composition and resulting properties, elements and compounds share several fundamental similarities. Both are built from atoms, participate in chemical reactions, obey conservation laws, and exist in various physical states. Understanding these similarities is key to a comprehensive understanding of chemistry and the material world. While the diversity of elements and compounds contributes to the rich tapestry of matter, their shared fundamental characteristics reveal a powerful underlying unity that binds them together. This understanding helps us to predict and understand the behavior of matter in a myriad of contexts, from the formation of stars to the complex reactions occurring within living organisms. The study of both elements and compounds, therefore, provides an indispensable foundation for the broader exploration of chemical science and the natural world.
Latest Posts
Latest Posts
-
Why Doesnt Christian Bale Remove His Mole
Jul 02, 2025
-
Somebody Once Told Me That The World Was Macaroni Lyrics
Jul 02, 2025
-
How Old Are You If Born In 1993
Jul 02, 2025
-
What Is 1 4 Of 1 4 Cup
Jul 02, 2025
-
Is Keri Russell Related To Kurt Russell
Jul 02, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements And Compounds Alike . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.