How Are Elements Arranged In The Modern Periodic Table
Kalali
Mar 12, 2025 · 7 min read
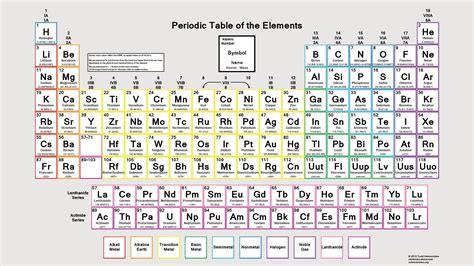
Table of Contents
How Are Elements Arranged in the Modern Periodic Table?
The modern periodic table, a cornerstone of chemistry, is a beautifully organized arrangement of chemical elements. Its structure isn't arbitrary; it's a reflection of the fundamental properties and behaviors of atoms, reflecting their electronic configurations and resulting chemical characteristics. Understanding the arrangement is key to understanding chemistry itself. This article delves deep into the principles behind the table's organization, exploring the history, the key organizing principles, and the information it conveys.
A Brief History: From Mendeleev to the Modern Table
The periodic table wasn't conjured overnight. Its development spans decades, culminating in the elegant structure we use today. While Dmitri Mendeleev is often credited with its creation, his 1869 table was just a pivotal step in a longer journey. Earlier attempts, notably those by Johann Wolfgang Döbereiner (with his triads) and John Newlands (with his Law of Octaves), hinted at underlying patterns in elemental properties but lacked the predictive power and comprehensive scope of Mendeleev's table.
Mendeleev's genius lay not just in organizing the known elements by atomic weight (the best approximation available at the time), but also in leaving gaps for elements yet to be discovered. He boldly predicted the properties of these missing elements based on the trends in his table – a testament to the power of his organization. These predictions were later spectacularly confirmed, solidifying the table's validity.
However, Mendeleev's table wasn't perfect. Some elements didn't perfectly fit the atomic weight-based ordering. This discrepancy was resolved with the discovery of atomic number, the number of protons in an atom's nucleus. Henry Moseley's work in the early 20th century showed that arranging elements by atomic number, rather than atomic weight, resolved the inconsistencies and provided a more accurate reflection of their chemical properties. This refinement led to the modern periodic table we know today.
The Key Organizing Principles: Atomic Number and Electronic Configuration
The modern periodic table is fundamentally organized by atomic number, which directly reflects the number of protons in an atom's nucleus. This number uniquely identifies each element. However, the table's structure also elegantly reflects the electronic configuration of the elements – the arrangement of electrons in their energy levels or shells. This is the crucial link between atomic number and the periodic trends observed.
Energy Levels and Electron Shells
Electrons are arranged in energy levels or shells around the nucleus. Each shell can only hold a specific number of electrons. The first shell (closest to the nucleus) holds a maximum of two electrons, the second shell eight, and so on. This arrangement dictates the element's chemical reactivity and bonding behavior.
Valence Electrons: The Key to Chemical Reactivity
The valence electrons are the electrons in the outermost shell. These electrons are the ones most involved in chemical bonding, determining the element's reactivity. Elements with similar numbers of valence electrons exhibit similar chemical properties, forming the basis of the periodic table's columns or groups.
The Structure of the Modern Periodic Table: Rows, Columns, and Blocks
The periodic table is a two-dimensional array with elements arranged in rows (periods) and columns (groups). The arrangement reflects both the increasing atomic number and the filling of electron shells.
Periods (Rows): Reflecting Electron Shells
Each period corresponds to a principal energy level or shell. As we move across a period, we add one proton and one electron, filling the electron shells sequentially. For instance, the first period contains only hydrogen and helium, as their electrons fill the first shell. The second period elements fill the second shell, and so on. The number of elements in each period increases as we go down the table because higher energy levels can accommodate more electrons.
Groups (Columns): Reflecting Valence Electrons and Chemical Properties
Elements in the same group or column have the same number of valence electrons and, consequently, share similar chemical properties. For example, Group 1 (alkali metals) all have one valence electron, resulting in similar reactivity, such as readily losing that electron to form +1 ions. Group 18 (noble gases) have full valence shells (except helium, which has a full first shell), making them exceptionally unreactive.
Blocks: Reflecting the Filling of Subshells
The periodic table is further divided into blocks based on the subshell (s, p, d, f) that the last electron enters.
- s-block: This block encompasses Groups 1 and 2 (alkali and alkaline earth metals). The last electron enters an s subshell.
- p-block: This block includes Groups 13 to 18. The last electron enters a p subshell. This block includes a wide variety of elements, showcasing diverse properties.
- d-block: This block constitutes the transition metals, occupying Groups 3 to 12. The last electron enters a d subshell. Transition metals are known for their variable oxidation states and complex ion formation.
- f-block: This block, often placed separately at the bottom of the main table, contains the lanthanides (rare earth elements) and actinides. The last electron enters an f subshell. These elements exhibit similar chemical properties within their respective series.
Periodic Trends: A Consequence of Electronic Structure
The arrangement of elements in the periodic table leads to predictable trends in their physical and chemical properties. These trends are a direct consequence of the increasing atomic number and the resulting changes in electronic configuration. Understanding these trends is crucial for predicting the behavior of elements and compounds.
Atomic Radius: Size Matters
Atomic radius generally increases down a group and decreases across a period. Going down a group, additional electron shells are added, increasing the atom's size. Across a period, the increased nuclear charge pulls the electrons closer, resulting in a smaller atomic radius.
Ionization Energy: The Energy to Remove an Electron
Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period (due to increasing nuclear charge) and decreases down a group (due to increased distance from the nucleus and shielding effects).
Electronegativity: The Pull on Electrons in a Bond
Electronegativity measures an atom's ability to attract electrons in a chemical bond. It generally increases across a period and decreases down a group, mirroring the trends in ionization energy.
Electron Affinity: The Attraction for an Additional Electron
Electron affinity refers to the energy change when an atom gains an electron. Trends are less clear-cut than ionization energy and electronegativity but generally follow similar patterns.
Beyond the Basics: Further Refinements and Applications
The modern periodic table is a constantly evolving tool. Ongoing research continues to refine our understanding of element properties and relationships. For example, the inclusion of the f-block elements, the detailed understanding of isotopic variations, and the exploration of synthetic elements have all enriched the table.
The periodic table is not just an academic curiosity; it's a powerful predictive tool in chemistry, materials science, and many other fields. Its structure allows scientists to:
- Predict the properties of undiscovered elements: Just as Mendeleev did, scientists can use the periodic table to predict the properties of new elements based on their position within the table.
- Design new materials: The periodic table is essential in designing materials with specific properties, such as high strength, conductivity, or reactivity.
- Understand chemical reactions: The periodic table helps to understand why and how certain chemical reactions occur, facilitating the design of chemical processes and reactions.
- Develop new technologies: Advancements in various fields, including electronics, medicine, and energy, rely heavily on understanding the properties of elements and their interactions, knowledge gained from studying the periodic table.
Conclusion: A Powerful Tool for Understanding the Universe
The modern periodic table is a testament to the power of scientific observation, prediction, and refinement. Its organization, based on atomic number and electronic configuration, beautifully reflects the fundamental principles governing the behavior of matter. This elegantly structured arrangement is not simply a chart; it's a dynamic tool, a roadmap to understanding the properties and interactions of elements, and a key to unlocking future scientific advancements. From its humble beginnings to its current sophisticated form, the periodic table stands as a powerful symbol of humanity's quest to comprehend and harness the universe's fundamental building blocks.
Latest Posts
Latest Posts
-
Why Did Jerry Thompson And Kelly Nelon Divorce
Jun 30, 2025
-
Allen And Roth Patio Furniture Replacement Parts
Jun 30, 2025
-
How Many Minutes Are In 20 Miles
Jun 30, 2025
-
How Many Days Is 72 Hours From Tuesday
Jun 30, 2025
-
How Many Cups Are In 3 Quarts Of Water
Jun 30, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements Arranged In The Modern Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.