How Are Elements In The Modern Periodic Table Arranged
Kalali
Mar 17, 2025 · 6 min read
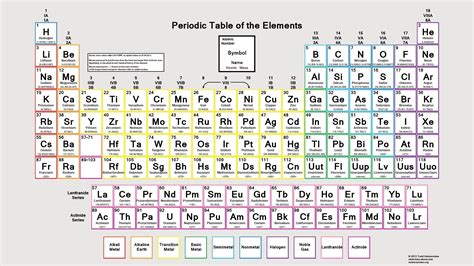
Table of Contents
How Are Elements in the Modern Periodic Table Arranged?
The modern periodic table, a cornerstone of chemistry, isn't just a random arrangement of elements. Its structured organization reflects the fundamental properties of atoms and allows us to predict the behavior of elements with remarkable accuracy. Understanding this arrangement is key to grasping the principles of chemistry and its applications. This article delves deep into the logic behind the table's structure, exploring the historical context, the underlying principles, and the significance of its organization.
From Mendeleev's Vision to the Modern Table
The periodic table we use today is a refined version of Dmitri Mendeleev's original table, published in 1869. Mendeleev, a Russian chemist, noticed patterns in the properties of elements when arranged by increasing atomic weight. He cleverly left gaps in his table, predicting the existence and properties of undiscovered elements based on the periodic trends he observed. These predictions were later verified, solidifying the table's power and accuracy.
The Key to Arrangement: Atomic Number
While Mendeleev used atomic weight, the modern periodic table's organization is fundamentally based on atomic number. The atomic number represents the number of protons in an atom's nucleus, which uniquely identifies an element. This is crucial because the number of protons determines the element's chemical properties. Isotopes, atoms of the same element with varying numbers of neutrons, have different atomic weights but identical chemical behaviors due to their shared atomic number.
The Structure: Periods and Groups
The periodic table is a grid-like structure organized into rows (periods) and columns (groups or families). This arrangement reveals the repeating patterns of elemental properties.
Periods: Reflecting Electron Shells
Each row, or period, represents a principal energy level, or electron shell. As you move across a period, you add one proton and one electron, gradually filling the electron shells. Elements within the same period have the same number of electron shells but differing numbers of electrons in the outermost shell, the valence shell. The number of electrons in the valence shell plays a crucial role in determining an element's chemical reactivity.
Example: The second period starts with Lithium (Li) and ends with Neon (Ne). All these elements have two electron shells, but the number of valence electrons increases from one (Li) to eight (Ne). This varying number of valence electrons accounts for the differences in their properties.
Groups: Reflecting Valence Electrons and Chemical Behavior
The columns, or groups, represent elements with similar valence electron configurations. Elements in the same group tend to exhibit similar chemical properties because they have the same number of electrons in their outermost shell, which determines their bonding behavior. This similarity in chemical behavior is a central feature of the periodic table.
Example: The elements in Group 1, the alkali metals (Li, Na, K, etc.), all have one valence electron. This makes them highly reactive, readily losing that electron to form +1 ions. Similarly, Group 18, the noble gases (He, Ne, Ar, etc.), have full valence shells, making them generally unreactive.
The Blocks: s, p, d, and f
Within the periodic table, elements are further categorized into blocks based on the electron subshells being filled:
-
s-block: This block includes Groups 1 and 2 (alkali and alkaline earth metals). Elements in this block have their outermost electrons in the s subshell.
-
p-block: This block encompasses Groups 13-18. Elements in this block have their outermost electrons in the p subshell. This block includes a diverse range of elements, from nonmetals to metalloids to some metals.
-
d-block: This block, also known as the transition metals, comprises Groups 3-12. Elements in this block have their outermost electrons filling the d subshell. Transition metals are known for their variable oxidation states and often form colored compounds.
-
f-block: This block, situated separately at the bottom of the table, includes the lanthanides and actinides. Elements in this block have their outermost electrons filling the f subshell. Many f-block elements are radioactive.
Periodic Trends: Unveiling Patterns in Properties
The arrangement of the periodic table allows us to predict and understand various periodic trends, which are systematic changes in properties as you move across periods or down groups. These trends include:
-
Atomic Radius: Atomic radius generally increases down a group (due to added electron shells) and decreases across a period (due to increased nuclear charge).
-
Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally decreases down a group and increases across a period.
-
Electron Affinity: The energy change associated with adding an electron to an atom. Electron affinity generally increases across a period and shows less consistent trends down a group.
-
Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
-
Metallic Character: The tendency of an element to lose electrons and form positive ions. Metallic character generally increases down a group and decreases across a period.
Understanding these trends helps us predict the reactivity and bonding behavior of elements.
The Importance of the Periodic Table
The periodic table is more than just a chart; it's a powerful tool that underpins much of our understanding of chemistry. Its significance stems from its ability to:
-
Predict the Properties of Elements: Based on its position, we can predict many of an element's properties, including its reactivity, bonding behavior, and physical state.
-
Organize Chemical Information: It provides a systematic way to organize and access information about the vast number of elements.
-
Understand Chemical Reactions: The table helps us understand why certain reactions occur and predict the products formed.
-
Develop New Materials: The periodic table guides the search for new materials with specific properties by allowing scientists to strategically combine elements.
Beyond the Basics: Further Refinements and Applications
The modern periodic table continues to evolve. While the core structure remains consistent, ongoing research, especially in the realm of superheavy elements, leads to refinements and additions. The discovery and characterization of new elements constantly test and expand our understanding of periodic trends.
The applications of the periodic table extend far beyond the classroom. It's crucial in various fields, including:
-
Materials Science: Designing new materials with specific properties, such as strength, conductivity, or reactivity.
-
Medicine: Understanding the interactions of elements with biological systems and developing new drugs and treatments.
-
Environmental Science: Analyzing the behavior of elements in the environment and addressing pollution issues.
-
Nuclear Science: Understanding nuclear reactions and developing new energy sources.
Conclusion: A Powerful Tool for Understanding the Universe
The periodic table's arrangement, based on atomic number and electron configuration, is a testament to the elegance and predictability of the natural world. Its structure reveals fundamental patterns in elemental properties, facilitating predictions about chemical behavior and paving the way for countless scientific advancements. From predicting the existence of undiscovered elements to guiding the design of new materials, the periodic table remains an indispensable tool for chemists and scientists worldwide, a true cornerstone of scientific understanding. Its enduring legacy lies not only in its organization but in its ability to continuously illuminate the intricate workings of matter and the universe itself. Understanding its structure is crucial for anyone seeking a deeper appreciation of the fundamental principles that govern chemistry and the natural world.
Latest Posts
Latest Posts
-
What Is 1 5 8 As A Decimal
Mar 17, 2025
-
3 Feet 6 Inches In Cm
Mar 17, 2025
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Are Elements In The Modern Periodic Table Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
