How Are The Elements In The Modern Periodic Table Arranged
Kalali
Mar 17, 2025 · 6 min read
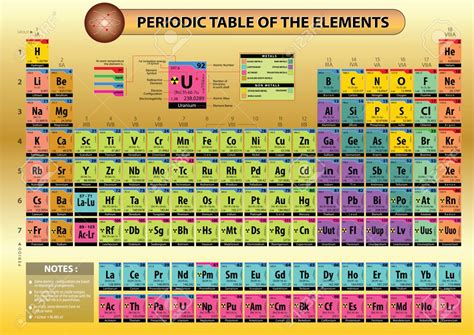
Table of Contents
How Are the Elements in the Modern Periodic Table Arranged?
The modern periodic table, a cornerstone of chemistry, isn't just a random assortment of elements. Its meticulous arrangement reflects the fundamental properties and behaviors of atoms, revealing patterns and relationships that underpin our understanding of the material world. This arrangement, far from arbitrary, is based on the atomic number, electron configuration, and resulting periodic trends in properties. Understanding this organization unlocks the secrets of chemical reactivity, bonding, and the vast diversity of matter around us.
The Foundation: Atomic Number and Electron Configuration
The key to understanding the periodic table's structure lies in the atomic number, which represents the number of protons in an atom's nucleus. This number uniquely identifies each element. Crucially, the number of protons also dictates the number of electrons in a neutral atom, and it's the arrangement of these electrons that governs an element's chemical behavior.
Electrons occupy specific energy levels, or shells, surrounding the nucleus. These shells are further subdivided into subshells (s, p, d, and f), each capable of holding a specific number of electrons. The electron configuration describes the distribution of electrons across these shells and subshells. For example, hydrogen (atomic number 1) has one electron in the 1s subshell, while helium (atomic number 2) has two electrons in the 1s subshell. This seemingly simple difference leads to vastly different chemical properties.
The Significance of Electron Shells and Subshells
The outermost shell, known as the valence shell, contains the valence electrons. These electrons are the primary participants in chemical bonding and reactions. Elements with similar valence electron configurations tend to exhibit similar chemical properties. This fundamental principle is the bedrock of the periodic table's organization.
The filling of electron shells follows specific rules, determined by the Aufbau principle and Hund's rule. The Aufbau principle states that electrons fill the lowest energy levels first. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. These rules determine the electron configurations and ultimately the placement of elements on the periodic table.
The Structure of the Periodic Table: Periods and Groups
The periodic table is arranged in a grid-like format with rows and columns. The rows are called periods, and the columns are called groups or families.
Periods: Reflecting Electron Shells
Each period corresponds to a principal energy level (shell) in an atom. The first period has only two elements (hydrogen and helium) because the first energy level can only hold a maximum of two electrons. As we move down the table to subsequent periods, the number of elements increases because higher energy levels can accommodate more electrons. The increased number of electrons in each successive period results in greater complexity in the chemical properties of the elements. Each period begins with an element that has a new electron shell being filled and ends with a noble gas, which has a complete outermost shell.
Groups: Sharing Valence Electrons and Chemical Properties
The groups are the vertical columns in the periodic table. Elements within the same group have the same number of valence electrons and therefore exhibit similar chemical properties. This similarity stems from the fact that their valence electrons participate in chemical bonding in similar ways.
Key Groups and their Characteristics:
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron. They readily lose this electron to form +1 ions.
- Group 2 (Alkaline Earth Metals): Reactive metals with two valence electrons. They tend to lose these electrons to form +2 ions.
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons. They readily gain one electron to form -1 ions.
- Group 18 (Noble Gases): Inert gases with a complete valence shell (eight electrons, except for helium with two). Their stability makes them unreactive.
- Transition Metals: Occupying the central block of the table, these elements have partially filled d orbitals and exhibit variable oxidation states, leading to a wide range of chemical properties and complex compounds.
- Inner Transition Metals (Lanthanides and Actinides): Placed separately at the bottom of the table, these elements have partially filled f orbitals and demonstrate complex chemical behaviors.
Periodic Trends: Predicting Properties
The arrangement of the periodic table allows us to predict certain trends in the properties of elements. These trends are crucial for understanding chemical reactivity and bonding. These trends include:
- Atomic Radius: The size of an atom generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increased nuclear charge pulling electrons closer).
- Ionization Energy: The energy required to remove an electron from an atom. Ionization energy generally decreases down a group (outer electrons are further from the nucleus) and increases across a period (increased nuclear charge holds electrons more tightly).
- Electron Affinity: The energy change that occurs when an atom gains an electron. Electron affinity generally increases across a period and decreases down a group.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Electronegativity generally increases across a period and decreases down a group.
Understanding these periodic trends allows chemists to predict the reactivity and bonding behavior of elements, aiding in the design of new materials and the synthesis of new compounds.
Beyond the Basic Arrangement: Subtleties and Exceptions
While the basic structure of the periodic table is straightforward, there are some subtleties and exceptions to the general trends. These exceptions often arise from the complex interactions between electrons and the nucleus, as well as the relativistic effects in heavier elements. For example, some anomalies in ionization energy and electron affinity are observed due to electron-electron repulsions or the stability associated with half-filled or completely filled subshells.
The periodic table's elegance also extends to predicting the physical states of matter at standard conditions. Metals are predominantly found on the left side of the table, while nonmetals are located on the right. Metalloids, exhibiting properties of both metals and nonmetals, form a diagonal boundary between the two.
The Periodic Table's Ongoing Evolution
The periodic table, far from being a static entity, continues to evolve. With the ongoing synthesis of new elements and the advancement of our understanding of atomic structure and chemical behavior, our knowledge of the periodic trends and relationships between elements will continue to refine our understanding and application of this powerful tool. The discovery and characterization of new superheavy elements will inevitably lead to further adjustments and refinements within the table's framework.
Conclusion: A Powerful Tool for Understanding the Material World
The modern periodic table is more than just a chart; it's a powerful tool that encapsulates our understanding of the fundamental building blocks of matter. Its arrangement, based on atomic number, electron configuration, and periodic trends, provides a framework for predicting and understanding the vast array of chemical and physical properties of elements. From the reactivity of alkali metals to the inertness of noble gases, the periodic table offers a systematic way to organize and comprehend the complex world of chemistry, driving innovation and discovery across numerous scientific disciplines. The periodic table's ongoing evolution underscores the dynamic nature of scientific exploration and its enduring relevance in unveiling the secrets of the universe. The table's design, reflecting fundamental atomic structures and chemical properties, remains a testament to human ingenuity and our continuous quest to understand the natural world.
Latest Posts
Latest Posts
-
Which Is Greater 0 25 Or 0 5
Mar 17, 2025
-
How Many Ml In 25 Ounces
Mar 17, 2025
-
How Is Humidity Related To Air Pressure
Mar 17, 2025
-
How Many Eggs Does A Leopard Gecko Lay
Mar 17, 2025
-
How Many Calories Is In A Gram Of Uranium
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Are The Elements In The Modern Periodic Table Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
