How Did Rutherford Know That The Nucleus Was Positively Charged
Kalali
Mar 31, 2025 · 5 min read
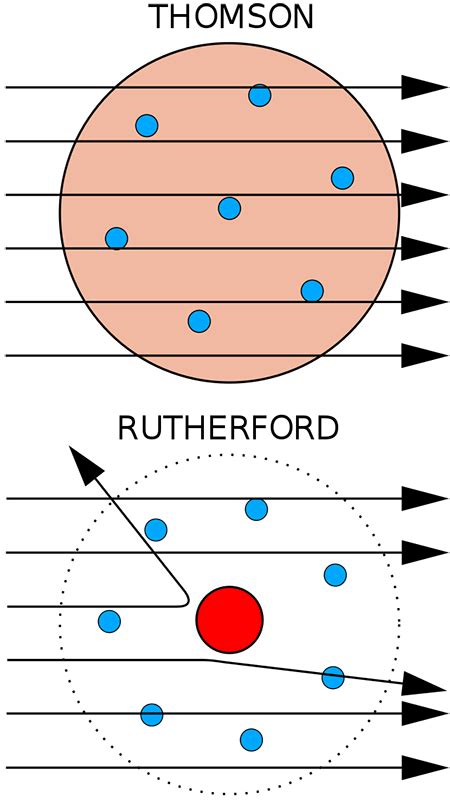
Table of Contents
- How Did Rutherford Know That The Nucleus Was Positively Charged
- Table of Contents
- How Did Rutherford Know That the Nucleus Was Positively Charged?
- The Pre-Rutherford Atomic Model: A Necessary Context
- The Geiger-Marsden Experiment: A Crucial Turning Point
- Interpreting the Results: The Birth of the Nuclear Model
- Further Evidence Supporting a Positively Charged Nucleus
- The Refinements and Subsequent Developments
- The Significance of Rutherford's Work: A Lasting Legacy
- Latest Posts
- Latest Posts
- Related Post
How Did Rutherford Know That the Nucleus Was Positively Charged?
Ernest Rutherford's groundbreaking discovery of the atomic nucleus and its positive charge revolutionized our understanding of the atom. This wasn't a sudden "eureka!" moment, but rather the culmination of meticulous experimentation, insightful interpretation, and a brilliant synthesis of existing scientific knowledge. This article delves into the experimental evidence and the logical reasoning that led Rutherford to conclude that the nucleus, the atom's dense central core, carried a positive electrical charge.
The Pre-Rutherford Atomic Model: A Necessary Context
Before understanding Rutherford's work, it's crucial to grasp the prevailing atomic model at the time – the "plum pudding" model proposed by J.J. Thomson. Thomson, who discovered the electron, envisioned the atom as a positively charged sphere with negatively charged electrons embedded within it, like plums in a pudding. This model, while a step forward from Dalton's indivisible atom, lacked a crucial element: a concentrated positive charge.
Thomson's model couldn't explain certain experimental observations, particularly the results of scattering experiments involving alpha particles. Alpha particles, positively charged particles emitted by certain radioactive elements, were becoming increasingly important tools in probing the structure of matter. This is where Rutherford's genius came into play.
The Geiger-Marsden Experiment: A Crucial Turning Point
Rutherford, along with his students Hans Geiger and Ernest Marsden, conducted a series of experiments now famously known as the Geiger-Marsden experiment, or the gold foil experiment. This experiment involved bombarding a thin gold foil with a beam of alpha particles. Surrounding the gold foil was a zinc sulfide screen that would flash when struck by an alpha particle, allowing the researchers to detect the scattering pattern.
The Expected Results (Based on the Plum Pudding Model): According to Thomson's plum pudding model, the alpha particles, with their relatively high mass and energy, were expected to pass straight through the gold foil with only minor deflections. The positive charge was assumed to be spread uniformly throughout the atom, providing only weak repulsive forces.
The Unexpected Results: A Shocking Discovery: The actual results were completely unexpected. While most alpha particles did pass through with minimal deflection, a small but significant number were scattered at large angles, some even bouncing almost directly back towards the source. This was astonishing. Imagine firing bullets at a tissue paper and having some of them bounce back!
Interpreting the Results: The Birth of the Nuclear Model
The large-angle scattering of alpha particles couldn't be explained by the plum pudding model. Rutherford realized that the only way to account for such significant deflections was to postulate the existence of a small, dense, positively charged region within the atom. This region, which he termed the "nucleus," was responsible for the strong repulsive forces that scattered the alpha particles.
The Logic: The immense force required to deflect an alpha particle through a large angle could only be generated by a highly concentrated positive charge. The vast majority of alpha particles passed through undeflected, implying that the atom is mostly empty space. Only when an alpha particle came close to this dense, positively charged region did it experience a strong repulsive force leading to significant deflection.
Further Evidence Supporting a Positively Charged Nucleus
Rutherford's conclusion wasn't based solely on the gold foil experiment. Several other lines of evidence converged to support the notion of a positively charged nucleus:
- Radioactive decay: The emission of alpha particles (positively charged) and beta particles (negatively charged) from radioactive nuclei suggested the presence of a complex internal structure within the atom with different charged components.
- Chemical properties: The periodic table, with its arrangement based on the chemical properties of elements, hinted at a fundamental structure within atoms that determined their reactivity. This structure couldn't be explained by the plum pudding model but was consistent with the concept of a positively charged nucleus surrounded by electrons.
- Atomic spectroscopy: The discrete spectral lines emitted by atoms when excited suggested quantized energy levels, which would later be explained by the Bohr model incorporating Rutherford's nuclear concept. This implied that electrons are orbiting the nucleus, not randomly distributed as per Thomson's model.
The Refinements and Subsequent Developments
Rutherford's nuclear model, while revolutionary, wasn't perfect. It couldn't explain the stability of the atom; according to classical physics, orbiting electrons should lose energy and spiral into the nucleus. This problem was addressed by Niels Bohr's model, which incorporated quantum mechanics to explain the stability of electron orbits.
Nevertheless, Rutherford's discovery laid the foundation for modern atomic theory. His brilliant interpretation of the Geiger-Marsden experiment marked a paradigm shift in our understanding of the atom. The realization that the nucleus was positively charged was crucial in paving the way for further advancements in nuclear physics and our understanding of the fundamental building blocks of matter.
The Significance of Rutherford's Work: A Lasting Legacy
Rutherford's work extends far beyond just the discovery of the nucleus. His meticulous experimental approach, his insightful interpretation of data, and his ability to synthesize diverse lines of evidence are hallmarks of great scientific inquiry. His legacy extends to the numerous advancements that followed, including:
- Nuclear physics: The development of nuclear physics as a distinct field of study was directly attributable to Rutherford's work. His discoveries laid the groundwork for understanding nuclear reactions, radioactivity, and the structure of the nucleus itself.
- Particle physics: The study of fundamental particles, including protons and neutrons, owes a debt to Rutherford's research, which demonstrated the need for a deeper understanding of the constituents of the nucleus.
- Chemistry: Our understanding of chemical bonding and reactivity is directly linked to our knowledge of atomic structure, which was revolutionized by Rutherford’s insights.
- Technology: Advancements in nuclear medicine, nuclear energy, and numerous other technologies are ultimately rooted in the fundamental discoveries made by Rutherford and his colleagues.
Rutherford’s contribution to our understanding of the atom, and specifically his determination of the nucleus' positive charge, represents a crucial milestone in scientific history. His legacy continues to inspire generations of scientists and underscores the power of rigorous experimentation, creative thinking, and the relentless pursuit of knowledge. The story of how Rutherford knew the nucleus was positively charged is not just a historical account but a testament to the scientific method itself—a powerful tool for unraveling the mysteries of the universe.
Latest Posts
Latest Posts
-
Calories In 1 Gram Of Uranium
Apr 02, 2025
-
The Use Of Force To Move An Object Is
Apr 02, 2025
-
How Long Does Ice Take To Melt
Apr 02, 2025
-
180 Out Of 240 As A Percentage
Apr 02, 2025
-
Greatest Common Multiple Of 6 And 15
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Did Rutherford Know That The Nucleus Was Positively Charged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
