How Do Lipids Differ From Other Organic Compounds
Kalali
Mar 15, 2025 · 6 min read
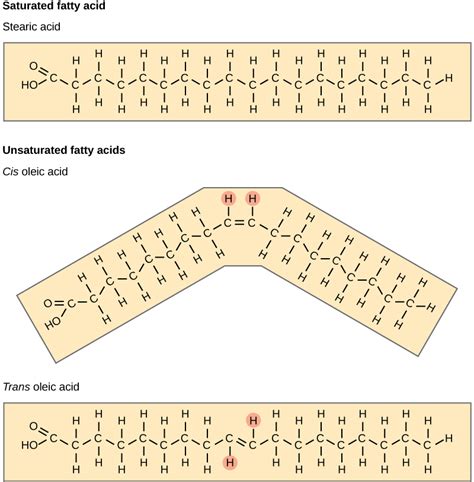
Table of Contents
How Do Lipids Differ From Other Organic Compounds?
Lipids, a diverse group of organic compounds, are often misunderstood and frequently grouped together with carbohydrates and proteins. While all three are essential for life and built from carbon, hydrogen, and oxygen, lipids possess unique structural and functional characteristics that set them apart. This comprehensive article will delve into the specific properties of lipids, contrasting them with other organic molecules to highlight their distinct nature and biological significance.
The Defining Characteristics of Lipids
Unlike carbohydrates and proteins, lipids are defined not by a specific monomeric unit or repeating structure but by their hydrophobicity. This means they are largely insoluble in water due to their predominantly nonpolar hydrocarbon chains. This hydrophobic nature stems from the high proportion of nonpolar C-H bonds compared to polar functional groups. This fundamental characteristic dictates their unique roles in biological systems.
1. Insolubility in Water: The Hallmark of Lipids
The nonpolar nature of lipids makes them insoluble in water, a crucial distinction from carbohydrates and proteins. Carbohydrates and proteins often contain polar functional groups like hydroxyl (-OH), carboxyl (-COOH), and amino (-NH2) groups, facilitating hydrogen bonding with water molecules and ensuring solubility. Lipids, however, lack these abundant polar groups, leading to their characteristic insolubility. This property contributes significantly to their function as structural components of cell membranes and energy storage molecules.
2. Diverse Chemical Structures: A Spectrum of Lipid Types
Another key difference lies in the structural diversity of lipids. While carbohydrates are primarily composed of monosaccharides linked to form polysaccharides, and proteins are linear chains of amino acids, lipids encompass a broad spectrum of molecular structures. These include:
-
Fatty Acids: These are long hydrocarbon chains with a carboxyl group at one end. They can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (multiple double bonds), influencing their physical properties and biological effects.
-
Triglycerides: These are the most common type of lipid, formed by esterification of three fatty acids to a glycerol molecule. They serve as the primary energy storage form in animals.
-
Phospholipids: These are crucial components of cell membranes. They consist of a glycerol backbone linked to two fatty acids and a phosphate group, which is further linked to a polar head group. The amphipathic nature (possessing both hydrophobic and hydrophilic regions) of phospholipids allows them to form bilayers in aqueous environments, forming the foundation of cell membranes.
-
Steroids: These have a characteristic four-ring structure. Cholesterol, a crucial component of cell membranes and precursor to steroid hormones, is a prime example. Steroid hormones, like testosterone and estrogen, regulate various physiological processes.
-
Waxes: These are esters of long-chain fatty acids and long-chain alcohols. They are water-resistant and serve protective functions in plants and animals.
This structural variety contributes to the wide array of functions lipids perform.
Contrasting Lipids with Carbohydrates and Proteins
Let's delve deeper into the contrasts between lipids and other major organic macromolecules:
Lipids vs. Carbohydrates
| Feature | Lipids | Carbohydrates |
|---|---|---|
| Solubility | Insoluble in water | Mostly soluble in water |
| Monomer | No single monomer, diverse structures | Monosaccharides (e.g., glucose) |
| Function | Energy storage, membrane structure, hormones | Energy source, structural support |
| Polymerization | Typically not polymeric; some exceptions like triglycerides | Form polymers (polysaccharides) |
| Polarity | Predominantly nonpolar | Typically polar due to many hydroxyl groups |
Carbohydrates primarily serve as a readily available energy source for cells. They are water-soluble due to their numerous hydroxyl groups, forming hydrogen bonds with water. In contrast, lipids store energy more efficiently, providing a compact and long-term energy reserve.
Lipids vs. Proteins
| Feature | Lipids | Proteins |
|---|---|---|
| Solubility | Insoluble in water | Solubility varies, many are soluble |
| Monomer | No single monomer, diverse structures | Amino acids |
| Function | Energy storage, membrane structure, hormones | Enzymes, structural support, transport |
| Polymerization | Typically not polymeric; some exceptions like triglycerides | Form polymers (polypeptides) |
| Structure | Diverse structures: fatty acids, triglycerides, phospholipids, steroids | Linear chains of amino acids with complex folding |
Proteins, composed of amino acids linked via peptide bonds, play diverse roles as enzymes (catalyzing biochemical reactions), structural components (collagen), and transporters. Their solubility varies based on their amino acid sequence and three-dimensional structure. Lipids, while not directly involved in catalysis or acting as structural proteins, are essential components of cell membranes and hormone precursors, highlighting their distinct contributions to cellular function.
The Biological Roles of Lipids
The unique properties of lipids allow them to perform various crucial roles:
1. Energy Storage: A High-Energy Density Fuel
Lipids, particularly triglycerides, are the most efficient form of energy storage in animals. They store significantly more energy per gram than carbohydrates or proteins. This high energy density is due to the large number of C-H bonds, which release substantial energy upon oxidation. The hydrophobic nature of lipids allows them to be stored in a compact, anhydrous form, minimizing the need to store water alongside them.
2. Structural Components of Cell Membranes: The Foundation of Life
Phospholipids are the primary building blocks of cell membranes. Their amphipathic nature allows them to spontaneously form lipid bilayers in aqueous environments. The hydrophobic tails of the phospholipids cluster together within the bilayer, away from water, while the hydrophilic heads face the aqueous environment, creating a selective barrier that regulates the passage of substances into and out of the cell. Cholesterol, another lipid, contributes to the fluidity and stability of cell membranes.
3. Hormones and Signaling Molecules: Chemical Messengers
Steroid hormones, derived from cholesterol, act as chemical messengers regulating various physiological processes. These include testosterone (male sex hormone), estrogen (female sex hormone), and cortisol (stress hormone). These hormones bind to specific receptors within cells, triggering specific cellular responses. Other lipid-derived signaling molecules, such as prostaglandins and leukotrienes, play roles in inflammation and immune responses.
4. Insulation and Protection: A Protective Shield
Lipids play a crucial role in thermal insulation, particularly in animals inhabiting cold environments. The subcutaneous fat layer, composed primarily of triglycerides, acts as an insulator, reducing heat loss. Waxes, found in plant cuticles and animal fur, provide a waterproof barrier, protecting against desiccation and environmental stressors.
5. Vitamins and Coenzymes: Essential for Biochemical Reactions
Certain lipids function as vitamins or components of coenzymes. Vitamin A, D, E, and K are fat-soluble vitamins, playing essential roles in vision, calcium metabolism, antioxidant defense, and blood clotting, respectively. Coenzyme Q10, a lipid-soluble quinone, participates in electron transport in mitochondria, playing a crucial role in energy production.
Conclusion: The Unique World of Lipids
Lipids, while often overshadowed by carbohydrates and proteins, represent a crucial class of organic molecules with diverse structures and functions. Their defining characteristic – hydrophobicity – directly influences their roles in energy storage, membrane structure, hormone production, and other essential biological processes. Understanding the unique properties of lipids is vital for comprehending the intricate workings of living organisms and their interactions with the environment. The diverse nature of lipids continues to be an area of active research, revealing ever-more intricate details of their biological significance and highlighting their essential contributions to life's complex processes.
Latest Posts
Latest Posts
-
How Many Oz Is In 3 4 Cup
Mar 15, 2025
-
How Can You Separate Sugar And Water
Mar 15, 2025
-
Cuanto Son 40 Oz En Litros
Mar 15, 2025
-
5 And 1 2 As A Decimal
Mar 15, 2025
-
How Do You Convert From Moles To Liters
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Do Lipids Differ From Other Organic Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
