How Do You Know If A Reaction Is Redox
Kalali
Mar 16, 2025 · 5 min read
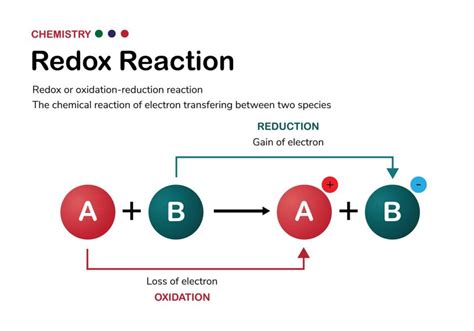
Table of Contents
How Do You Know if a Reaction is Redox? A Comprehensive Guide
Identifying redox reactions is crucial in chemistry, impacting various fields from electrochemistry to biology. Understanding the fundamental principles behind redox reactions—reactions involving the transfer of electrons—allows us to predict their behavior and utilize them effectively. This comprehensive guide will equip you with the knowledge and tools to confidently determine whether a chemical reaction is a redox reaction.
Understanding the Fundamentals: Oxidation and Reduction
Before delving into the identification process, let's solidify our understanding of the core concepts: oxidation and reduction. These terms, often abbreviated as "redox," are intrinsically linked and always occur simultaneously.
Oxidation: Loss of Electrons
Oxidation is the process where a species loses electrons. This loss of electrons leads to an increase in the oxidation state (oxidation number) of the atom involved. Remember the mnemonic OIL RIG: Oxidation Is Loss, Reduction Is Gain of electrons.
Example: Consider the reaction of magnesium (Mg) with oxygen (O₂):
2Mg(s) + O₂(g) → 2MgO(s)
Magnesium atoms lose two electrons each to become Mg²⁺ ions. This is an oxidation process.
Reduction: Gain of Electrons
Reduction is the process where a species gains electrons. This gain of electrons results in a decrease in the oxidation state of the atom.
Example: In the same magnesium-oxygen reaction, oxygen atoms gain electrons to form O²⁻ ions. This is a reduction process.
Oxidation States: The Key to Identification
Determining the oxidation states of atoms involved in a reaction is the most reliable method to identify a redox reaction. Oxidation states are hypothetical charges assigned to atoms in a molecule or ion, assuming that all bonds are completely ionic. Here are some rules to determine oxidation states:
- The oxidation state of an atom in its elemental form is 0. (e.g., Na, Cl₂, O₂)
- The oxidation state of a monatomic ion is equal to its charge. (e.g., Na⁺ = +1, Cl⁻ = -1)
- The oxidation state of hydrogen is +1, except in metal hydrides (e.g., NaH), where it is -1.
- The oxidation state of oxygen is usually -2, except in peroxides (e.g., H₂O₂) where it is -1 and in superoxides (e.g., KO₂) where it is -1/2.
- The sum of oxidation states of all atoms in a neutral molecule is 0.
- The sum of oxidation states of all atoms in a polyatomic ion is equal to the charge of the ion.
- Fluorine always has an oxidation state of -1.
- Other halogens usually have an oxidation state of -1, unless bonded to a more electronegative element like oxygen or another halogen.
Identifying Redox Reactions: A Step-by-Step Approach
Now let's put our knowledge into practice. Here's a systematic approach to identify redox reactions:
-
Assign oxidation states to all atoms in reactants and products. Carefully apply the rules mentioned above. This is the most crucial step.
-
Identify atoms that change their oxidation states. If no atom changes its oxidation state, it's not a redox reaction.
-
Determine which atoms are oxidized and which are reduced. Remember OIL RIG. An increase in oxidation state indicates oxidation, and a decrease indicates reduction.
-
Verify that the total number of electrons lost in oxidation equals the total number of electrons gained in reduction. This ensures the reaction is balanced in terms of electron transfer.
Example 1: A Redox Reaction
Consider the reaction between iron(II) ions and permanganate ions in acidic solution:
5Fe²⁺(aq) + MnO₄⁻(aq) + 8H⁺(aq) → 5Fe³⁺(aq) + Mn²⁺(aq) + 4H₂O(l)
-
Oxidation States:
- Fe²⁺: Fe = +2
- MnO₄⁻: Mn = +7, O = -2
- Fe³⁺: Fe = +3
- Mn²⁺: Mn = +2
- H⁺: H = +1
- H₂O: H = +1, O = -2
-
Oxidation State Changes: Iron's oxidation state increases from +2 to +3 (oxidation), while manganese's oxidation state decreases from +7 to +2 (reduction).
-
Oxidation and Reduction: Fe²⁺ is oxidized, and MnO₄⁻ is reduced.
-
Electron Balance: Fe²⁺ loses one electron per ion (5Fe²⁺ loses 5 electrons), and MnO₄⁻ gains 5 electrons. The electron transfer is balanced.
Therefore, this reaction is a redox reaction.
Example 2: A Non-Redox Reaction
Consider the neutralization reaction between hydrochloric acid and sodium hydroxide:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
-
Oxidation States:
- HCl: H = +1, Cl = -1
- NaOH: Na = +1, O = -2, H = +1
- NaCl: Na = +1, Cl = -1
- H₂O: H = +1, O = -2
-
Oxidation State Changes: No atom changes its oxidation state.
Therefore, this reaction is not a redox reaction.
Beyond Oxidation States: Other Indicators of Redox Reactions
While changes in oxidation states are the definitive test, some other indicators can suggest a redox reaction is occurring:
-
Presence of a strong oxidizing or reducing agent: Reactions involving known strong oxidizing agents (e.g., KMnO₄, K₂Cr₂O₇, HNO₃) or reducing agents (e.g., NaBH₄, LiAlH₄) are often redox reactions.
-
Gas evolution: The formation of gases like H₂, O₂, or Cl₂ often signifies a redox reaction, as these gases are often products of electron transfer processes.
-
Color change: Many redox reactions involve a change in color, due to changes in the oxidation states of transition metal ions. For example, the reduction of MnO₄⁻ (purple) to Mn²⁺ (colorless) results in a striking color change.
-
Change in temperature: Redox reactions often involve significant enthalpy changes, leading to a noticeable temperature increase (exothermic) or decrease (endothermic).
Common Mistakes to Avoid
-
Incorrectly assigning oxidation states: This is the most frequent mistake. Pay close attention to the rules and practice assigning oxidation states regularly.
-
Ignoring spectator ions: Spectator ions (ions that don't participate in the reaction) don't affect the redox nature of the reaction. Focus on the species that undergo oxidation state changes.
-
Not balancing the electron transfer: Ensure that the number of electrons lost equals the number of electrons gained.
Conclusion
Identifying redox reactions requires a systematic approach based on a thorough understanding of oxidation states and the principles of oxidation and reduction. While changes in oxidation states offer the most conclusive method, other indicators can provide supporting evidence. By carefully following the steps outlined above and practicing regularly, you'll confidently determine whether a chemical reaction involves the fascinating transfer of electrons—the hallmark of a redox reaction. Remember to always double-check your work and practice frequently to improve your skills in identifying these important chemical processes. The more you practice, the easier it becomes to recognize the subtle clues that indicate a redox reaction is taking place. This skill is crucial for understanding various chemical phenomena and processes in many scientific fields.
Latest Posts
Latest Posts
-
What Is 26 Out Of 30 As A Percentage
Mar 17, 2025
-
How Many Feet Is 26 In
Mar 17, 2025
-
What Is 6 Out Of 20 As A Percentage
Mar 17, 2025
-
What Is Melting Point Of Glass
Mar 17, 2025
-
Cuanto Es El 30 Por Ciento De 500
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Do You Know If A Reaction Is Redox . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
