How Do You Separate Sugar From Water
Kalali
Mar 19, 2025 · 5 min read
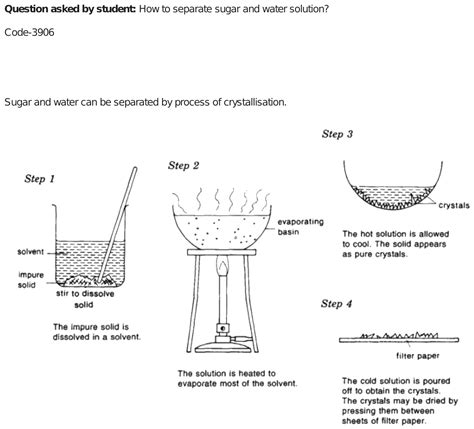
Table of Contents
How Do You Separate Sugar From Water? A Comprehensive Guide
Separating sugar from water might seem like a simple task, but understanding the underlying principles and exploring various methods reveals a fascinating interplay of chemistry and physics. This comprehensive guide delves into the science behind this separation and outlines several effective techniques, each with its own advantages and limitations. We'll explore everything from basic evaporation to more advanced techniques, helping you choose the best method depending on your needs and resources.
Understanding the Sugar-Water Solution
Before diving into separation methods, let's establish a foundational understanding of what we're dealing with: a homogeneous mixture. A sugar-water solution is a homogeneous mixture because the sugar (solute) dissolves completely in the water (solvent), creating a uniform composition throughout. The sugar molecules disperse evenly among the water molecules, forming a solution where individual components are indistinguishable to the naked eye. This homogeneous nature makes separating the components more challenging than separating a heterogeneous mixture, like sand and water.
Methods for Separating Sugar from Water
Several techniques can effectively separate sugar from water, each relying on different physical or chemical properties of the components. Let's explore some of the most common methods:
1. Evaporation
This is perhaps the simplest and most widely understood method. Evaporation leverages the difference in boiling points between water (100°C at standard pressure) and sugar (which decomposes before reaching its melting point of 186°C).
- Process: The sugar-water solution is heated gently. The water evaporates, leaving behind the sugar crystals. It's crucial to use a low heat to prevent the sugar from caramelizing (burning) before all the water evaporates. The process is complete when all the liquid has evaporated and only dry sugar remains.
- Advantages: Simple, readily accessible equipment (pot, stove), effective for small quantities.
- Disadvantages: Time-consuming, requires careful monitoring to avoid burning the sugar, may not be suitable for large-scale separations.
2. Distillation
Distillation is a more sophisticated technique than evaporation, particularly useful for separating liquids with relatively close boiling points. While less practical for simple sugar-water separation at home, it's a vital industrial process.
- Process: The sugar-water solution is heated in a distillation apparatus. The water vaporizes, is cooled and condensed, and collected separately. The sugar remains in the distillation flask.
- Advantages: Highly effective for separating liquids with similar boiling points, can produce pure water.
- Disadvantages: Requires specialized equipment (distillation apparatus), more complex than evaporation, not cost-effective for separating sugar from water on a small scale.
3. Chromatography
Chromatography, a powerful separation technique, is based on the differential affinity of components for a stationary phase and a mobile phase. While not typically used for sugar-water separation due to the simplicity of other methods, it's a versatile technique employed in various scientific and industrial applications.
- Process: Involves passing the sugar-water solution through a medium (stationary phase) where the sugar and water move at different speeds based on their interactions with the medium and a mobile phase (e.g., solvent).
- Advantages: Highly effective for separating complex mixtures, can resolve closely related compounds.
- Disadvantages: Requires specialized equipment and expertise, not cost-effective or practical for simple sugar-water separation.
4. Reverse Osmosis
Reverse osmosis is a membrane-based separation technique that uses pressure to force water molecules through a semipermeable membrane, leaving behind larger molecules like sugar. This method isn't typically used for sugar-water separation, as evaporation is generally simpler and cheaper. However, it’s relevant in contexts where water purification is prioritized.
- Process: A high pressure is applied to the sugar-water solution, forcing water molecules through the membrane. The sugar remains behind, concentrated in the retentate stream.
- Advantages: Effective for separating dissolved solids from water, can be used for large-scale separations.
- Disadvantages: Requires specialized equipment (high-pressure pumps, semipermeable membranes), relatively expensive, not usually the most practical method for this specific separation.
5. Fractional Crystallization
Fractional crystallization exploits the difference in solubility of substances at various temperatures. Although less practical than evaporation for sugar-water separation, it’s a useful technique for separating mixtures of solids dissolved in a solvent.
- Process: Involves cooling the solution slowly. As the solution cools, the solubility of sugar decreases, and sugar crystals begin to form and precipitate out of the solution. The crystals can be separated from the remaining solution through filtration.
- Advantages: Suitable for separating mixtures of solids dissolved in a solvent, can result in high purity of the separated components.
- Disadvantages: Requires careful control of temperature and cooling rate, time-consuming, not the most efficient method for sugar-water separation.
Choosing the Right Method
The optimal method for separating sugar from water depends on various factors, including the quantity of the solution, available resources, desired purity of the separated components, and time constraints. For small-scale separations, evaporation is generally the most convenient and cost-effective method. For large-scale or high-purity requirements, distillation or reverse osmosis might be more appropriate, though these options require specialized equipment and expertise.
Safety Precautions
Regardless of the chosen method, it's essential to prioritize safety:
- Evaporation: Always supervise the heating process closely to prevent the sugar from burning. Use appropriate heat-resistant cookware and pot holders.
- Distillation: Handle the glassware with care. Be aware of the hot surfaces and avoid contact with the boiling solution.
- Other Methods: Follow all safety guidelines specified for the equipment and chemicals involved in the chosen separation technique.
Conclusion
Separating sugar from water is a fundamental concept illustrating the principles of separating components from mixtures. While evaporation is the simplest and most practical method for everyday applications, understanding other techniques like distillation, chromatography, reverse osmosis, and fractional crystallization provides a wider perspective on the diverse methodologies available for separating mixtures based on their chemical and physical properties. The choice of method ultimately depends on the specific context and desired outcome. By understanding the underlying principles and taking appropriate safety precautions, you can effectively separate sugar from water using the most suitable technique for your needs.
Latest Posts
Latest Posts
-
Properties Of Rhombus Rectangle And Square
May 09, 2025
-
How Many Hours Is 133 Minutes
May 09, 2025
-
Cuantos Son 72 Grados Fahrenheit En Centigrados
May 09, 2025
-
How Many Hours Is 257 Minutes
May 09, 2025
-
4 Out Of 30 Is What Percent
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Do You Separate Sugar From Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.