How Do You Separate Water And Sugar
Kalali
Mar 21, 2025 · 6 min read
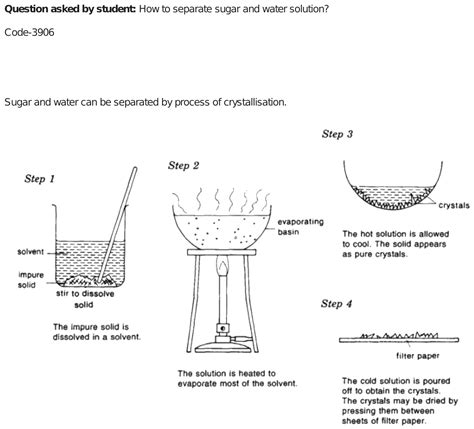
Table of Contents
How Do You Separate Water and Sugar? A Comprehensive Guide
Separating water and sugar is a fundamental concept in chemistry, crucial for understanding mixtures and the properties of matter. While seemingly simple, the process offers a fascinating glimpse into various separation techniques, highlighting the differences between mixtures and compounds. This comprehensive guide will explore several methods for separating water and sugar, explaining the scientific principles behind each and highlighting their advantages and disadvantages.
Understanding the Mixture: Water and Sugar
Before diving into separation techniques, it's essential to understand the nature of the water-sugar mixture. Sugar (sucrose) dissolves completely in water, forming a homogeneous mixture. This means the sugar molecules are evenly distributed throughout the water, and you can't visually distinguish them. Crucially, this is a physical mixture, not a chemical compound. The water and sugar retain their individual chemical identities; no new substance is formed. This distinction is vital because it determines the feasibility of different separation methods. We can exploit the differences in their physical properties (like boiling point and vapor pressure) to separate them.
Methods for Separating Water and Sugar
Several effective methods can separate water and sugar. Each method leverages different physical properties of the components. Let's explore the most common and effective approaches:
1. Evaporation
This is perhaps the most straightforward method. Evaporation relies on the difference in boiling points between water (100°C at standard pressure) and sugar (sugar decomposes before it boils).
Process:
- Heat the solution: Gently heat the water-sugar solution in a suitable container, such as a beaker or a saucepan. Avoid rapid boiling, which could cause splattering.
- Water evaporates: As the solution heats, the water will evaporate, turning into water vapor.
- Sugar remains: The sugar, having a much higher boiling point, will remain behind in the container as a solid residue.
- Crystallization (optional): For larger, purer sugar crystals, you can slow down the evaporation process. This allows for controlled crystallization, yielding larger sugar crystals.
Advantages:
- Simplicity: This method is straightforward and requires minimal equipment.
- Effectiveness: It effectively separates the water and sugar completely.
Disadvantages:
- Time-consuming: Evaporation can be a relatively slow process, especially with larger volumes of solution.
- Energy intensive: Heating the solution requires energy, making it less energy-efficient compared to other methods.
- Potential for sugar degradation: High temperatures could potentially caramelize the sugar, altering its properties.
2. Distillation
Distillation is a more refined technique that enhances the purity of the recovered water. It utilizes the difference in boiling points to separate the components of a liquid mixture.
Process:
- Boiling: The water-sugar solution is heated to boiling.
- Vaporization: The water turns into steam, leaving behind the sugar.
- Condensation: The steam is then passed through a condenser, where it cools and condenses back into liquid water.
- Collection: The purified water is collected in a separate container.
Advantages:
- High Purity: Distillation produces very pure water, free from dissolved sugar.
- Effective for multiple components: Distillation can be used to separate multiple volatile liquids with different boiling points.
Disadvantages:
- Complexity: Requires more sophisticated equipment, including a distillation apparatus.
- Energy Intensive: Similar to evaporation, it consumes considerable energy.
- Potential for loss: Some water may be lost during the process.
3. Reverse Osmosis
Reverse osmosis is a membrane-based separation technique that leverages pressure to separate water from dissolved substances, including sugar.
Process:
- Pressure application: High pressure is applied to the water-sugar solution.
- Membrane filtration: The solution is passed through a semi-permeable membrane that allows water molecules to pass through but blocks larger sugar molecules.
- Water separation: Pure water is collected on one side of the membrane, while the sugar solution remains on the other.
Advantages:
- High efficiency: Reverse osmosis is highly effective in removing dissolved solids from water.
- Energy-efficient (in some cases): While energy is needed for pressure, it can be more energy-efficient than distillation for large-scale operations.
Disadvantages:
- Expensive: Requires specialized equipment and membranes.
- Membrane fouling: Membranes can get clogged by the dissolved solids, reducing efficiency and lifespan.
- Not suitable for large sugar quantities: The membrane might not handle large amounts of sugar effectively.
4. Chromatography
Chromatography, particularly thin-layer chromatography (TLC) or paper chromatography, can be used to separate water and sugar, although it's less practical than evaporation or distillation for this specific task. Chromatography is typically used for separating complex mixtures of substances with differing polarities or affinities for a stationary phase.
Process (Conceptual for Water and Sugar):
A suitable stationary phase (e.g., filter paper) is used, and the water-sugar solution is applied as a spot. A solvent (mobile phase) is then used to move the components across the stationary phase. The difference in the interaction of water and sugar with the stationary phase would theoretically lead to separation; however, this difference is minor in practice.
Advantages:
- Useful for complex mixtures: Excellent for separating complex mixtures, especially in analytical chemistry.
Disadvantages:
- Impractical for large-scale separation of water and sugar: Not the most effective or efficient method for this specific separation.
- Requires specialized knowledge: This technique necessitates specific expertise and equipment.
Choosing the Right Method
The best method for separating water and sugar depends on several factors, including:
- Scale of separation: For small quantities, evaporation is often sufficient. For larger quantities, distillation or reverse osmosis might be more practical.
- Desired purity: If high-purity water is required, distillation is preferred.
- Available resources and equipment: The availability of equipment and resources dictates the feasibility of different methods.
- Energy considerations: Evaporation and distillation are energy-intensive, while reverse osmosis can be more efficient in some instances.
Safety Precautions
When performing these separations, especially evaporation and distillation, it's crucial to follow safety precautions:
- Adult supervision: Always have adult supervision, especially when working with heat.
- Appropriate glassware: Use heat-resistant glassware to avoid breakage.
- Proper ventilation: Ensure adequate ventilation to avoid inhaling any vapors.
- Eye protection: Wear safety glasses to protect your eyes from splashes or fumes.
- Heat-resistant gloves: Use heat-resistant gloves when handling hot containers.
Conclusion
Separating water and sugar is a simple yet instructive demonstration of fundamental separation techniques. Evaporation remains the simplest and most accessible method for small-scale separations, while distillation offers higher purity. Reverse osmosis provides an efficient approach for larger scales and high purity demands, although it requires more advanced equipment. Understanding the principles behind each method and choosing the most appropriate technique based on the specific needs is crucial for successful separation. Remember to always prioritize safety when conducting these experiments. The choice of method ultimately depends on the scale of the separation, the required purity of the separated components, and the available resources. Through careful consideration and adherence to safety guidelines, one can successfully separate water and sugar and gain a deeper understanding of chemical mixtures and separation techniques.
Latest Posts
Latest Posts
-
Does A Catfish Have A Backbone
Mar 28, 2025
-
How Many Feet Are In 42 Inches
Mar 28, 2025
-
How Many Acres Is A Square Mile
Mar 28, 2025
-
15 Out Of 50 As A Percentage
Mar 28, 2025
-
How Many Inches In 8 Cm
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Do You Separate Water And Sugar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
