How Is Bohr's Atomic Model Different From Rutherford's Model
Kalali
Mar 26, 2025 · 6 min read
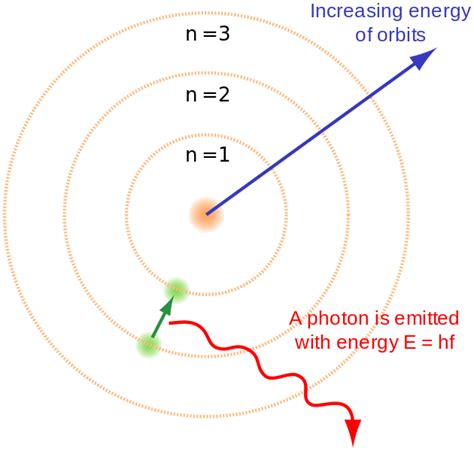
Table of Contents
How is Bohr's Atomic Model Different from Rutherford's Model? A Deep Dive into Atomic Structure
The journey of understanding the atom has been a fascinating odyssey, marked by groundbreaking models that refined our comprehension of this fundamental building block of matter. Two pivotal models, Rutherford's and Bohr's, stand out as crucial stepping stones in this journey. While Rutherford's model provided a crucial breakthrough, it was Bohr's model that significantly advanced our understanding, addressing limitations inherent in its predecessor. This article will meticulously dissect the differences between these two landmark models, exploring their strengths, weaknesses, and the revolutionary advancements that each represented.
Rutherford's Nuclear Model: A Revolutionary Leap
Ernest Rutherford's gold foil experiment in 1911 dramatically altered our perception of the atom. Before his work, the prevailing "plum pudding" model proposed by J.J. Thomson envisioned the atom as a uniform sphere of positive charge with negatively charged electrons embedded within. Rutherford's experiment, however, revealed a very different picture.
By bombarding a thin gold foil with alpha particles (positively charged helium nuclei), Rutherford observed that:
- Most alpha particles passed straight through the foil: This suggested that the atom is mostly empty space.
- A small fraction of alpha particles were deflected at large angles: This indicated the presence of a dense, positively charged center within the atom, which he termed the nucleus.
This led to Rutherford's nuclear model, which proposed that:
- The atom consists mostly of empty space.
- Almost all of the atom's mass and all of its positive charge are concentrated in a tiny, dense nucleus at the center.
- Electrons orbit the nucleus like planets orbiting the sun.
Strengths of Rutherford's Model:
- Explained the scattering of alpha particles: This was the model's primary triumph, successfully explaining the results of the gold foil experiment.
- Introduced the concept of the nucleus: This was a revolutionary concept that fundamentally changed our understanding of atomic structure.
Weaknesses of Rutherford's Model:
- Classical physics failure: According to classical electromagnetic theory, an orbiting electron should continuously emit electromagnetic radiation, losing energy and spiraling into the nucleus, causing the atom to collapse. This didn't align with the observed stability of atoms.
- Failed to explain atomic spectra: The model couldn't explain the discrete, line spectra observed when atoms emit light. Classical physics predicted a continuous spectrum.
- Didn't specify electron arrangement: The model offered no information about the arrangement or distribution of electrons within the atom.
Bohr's Atomic Model: Incorporating Quantum Theory
Niels Bohr's model, introduced in 1913, significantly improved upon Rutherford's by incorporating the newly emerging principles of quantum theory. Bohr addressed the key flaws of Rutherford's model by postulating several groundbreaking concepts:
- Quantized energy levels: Bohr proposed that electrons orbit the nucleus only in specific, discrete energy levels or shells, rather than a continuous range of orbits. These energy levels are quantized, meaning they can only have certain specific values.
- No radiation emission during stable orbits: Electrons in these stable orbits do not emit electromagnetic radiation, contrary to classical physics predictions. They only emit or absorb energy when transitioning between energy levels.
- Energy level transitions and spectral lines: The emission or absorption of energy corresponds to a specific frequency of light, explaining the discrete line spectra observed in atomic emission and absorption experiments. The energy difference between the levels dictates the frequency of the emitted or absorbed photon according to the equation: ΔE = hf, where ΔE is the energy difference, h is Planck's constant, and f is the frequency.
Bohr's model successfully predicted the spectral lines of hydrogen, a significant achievement that showcased the power of incorporating quantum principles into atomic theory.
Key Differences Between Rutherford's and Bohr's Models:
| Feature | Rutherford's Model | Bohr's Model |
|---|---|---|
| Electron Orbits | Continuous range of orbits | Discrete, quantized energy levels or shells |
| Electron Behavior | Electrons constantly radiate energy | Electrons do not radiate energy in stable orbits |
| Atomic Stability | Atom predicted to be unstable, collapsing | Atom is stable due to quantized energy levels |
| Atomic Spectra | Unable to explain discrete line spectra | Successfully explains the line spectra of hydrogen |
| Electron Arrangement | No specific electron arrangement described | Electrons occupy specific energy levels and subshells |
| Quantum Theory | Not incorporated | Incorporates key principles of quantum theory |
Strengths of Bohr's Model:
- Explained the hydrogen spectrum: This was a major success, validating the quantized energy levels and the concept of electron transitions.
- Introduced the concept of quantized energy levels: This was a groundbreaking contribution to quantum theory.
- Provided a simple picture of atomic structure: While not completely accurate, the model offered a relatively intuitive visual representation of the atom.
Weaknesses of Bohr's Model:
- Only worked for hydrogen: The model failed to accurately predict the spectra of atoms with more than one electron.
- Couldn't explain the intensities of spectral lines: The model didn't account for the varying intensities of the lines observed in spectra.
- Couldn't explain the fine structure of spectral lines: The model didn't account for the small splitting of spectral lines observed under high resolution.
- Didn't account for electron-electron interactions: The model didn't consider the interactions between electrons in multi-electron atoms.
- Classical physics limitations: The model still relied on classical concepts like circular orbits, which were ultimately superseded by more sophisticated quantum mechanical descriptions.
Beyond Bohr: The Quantum Mechanical Model
Bohr's model, while a significant step forward, was ultimately superseded by the more comprehensive quantum mechanical model developed in the 1920s. This model, based on the work of Schrödinger, Heisenberg, and others, utilizes the wave-particle duality of matter and the uncertainty principle to describe the behavior of electrons within atoms.
The quantum mechanical model significantly differs from Bohr's in that it:
- Replaces definite orbits with orbitals: Instead of definite paths, electrons occupy orbitals, which are regions of space where the probability of finding an electron is high.
- Describes electron behavior probabilistically: The model does not specify the exact location or momentum of an electron but rather describes the probability of finding it in a particular region.
- Accounts for electron-electron interactions: The quantum mechanical model successfully accounts for interactions between electrons in multi-electron atoms.
- Explains the fine structure of spectral lines: The model accurately predicts the small splitting of spectral lines.
Conclusion: A Legacy of Refinement
Rutherford's and Bohr's models represent landmark achievements in our understanding of atomic structure. While Rutherford's model provided the crucial foundation by introducing the concept of the nucleus, it was Bohr's incorporation of quantum theory that significantly advanced our comprehension of atomic behavior and successfully explained the hydrogen spectrum. Although Bohr's model had its limitations, it served as a vital bridge to the more complete and accurate quantum mechanical model, which ultimately replaced it. The journey of understanding the atom continues, but these early models remain crucial steps in that ongoing exploration. The development and refinement of atomic models illustrate the iterative nature of scientific progress, where each model builds upon its predecessors, addressing limitations and leading to a progressively deeper and more accurate understanding of the natural world.
Latest Posts
Latest Posts
-
Does Constant Velocity Mean No Acceleration
Mar 29, 2025
-
What Is A 16 Out Of 25
Mar 29, 2025
-
What Is The Percentage Of 12 Out Of 20
Mar 29, 2025
-
What Percent Of 200 Is 60
Mar 29, 2025
-
80 C Is What In Fahrenheit
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Is Bohr's Atomic Model Different From Rutherford's Model . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
