How Long Does Water Take To Evaporate
Kalali
Mar 10, 2025 · 5 min read
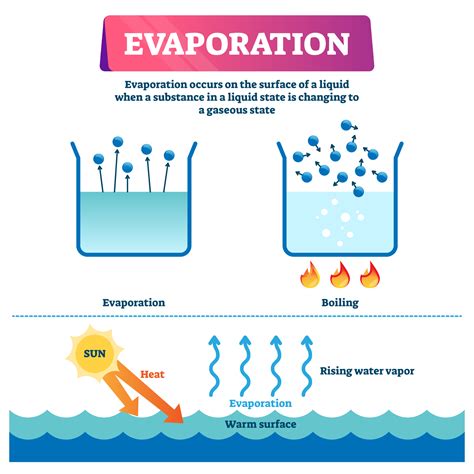
Table of Contents
How Long Does Water Take to Evaporate? A Comprehensive Guide
Evaporation, the transformation of water from a liquid to a gaseous state, is a fundamental process shaping our climate and environment. Understanding its timescale, however, is far from straightforward. It's not simply a matter of "X hours" or "Y days." The time it takes for water to evaporate depends on a complex interplay of several key factors. This comprehensive guide will delve into these factors, providing you with a nuanced understanding of this crucial process.
The Key Factors Influencing Evaporation Rate
Several environmental conditions significantly impact the rate at which water evaporates. These factors work in concert, often synergistically, to determine the overall evaporation time. Let's explore each one individually:
1. Temperature: The Driving Force
Temperature is arguably the most influential factor. Higher temperatures provide water molecules with more kinetic energy, allowing them to overcome the intermolecular forces holding them together in the liquid state and escape into the atmosphere as water vapor. Conversely, lower temperatures significantly slow down evaporation. A cold winter's day will see considerably slower evaporation than a hot summer's day. The relationship isn't linear, however; the rate of evaporation increases exponentially with temperature increases.
2. Humidity: The Atmospheric Barrier
Humidity, the amount of water vapor already present in the air, plays a crucial role. Air can only hold a certain amount of water vapor at a given temperature – its saturation point. High humidity means the air is already close to saturation, reducing the capacity for further evaporation. Think of it like a sponge – a nearly saturated sponge absorbs water much more slowly than a dry one. Low humidity, on the other hand, provides ample space for more water vapor, accelerating evaporation.
3. Wind Speed: The Accelerant
Wind acts as a crucial catalyst, removing the saturated air layer immediately above the water's surface. This constant replacement of saturated air with drier air allows for continuous evaporation. Strong winds dramatically increase evaporation rates, while calm conditions significantly slow it down. Imagine a fan blowing over a puddle – the puddle dries much faster due to the continuous removal of moisture-laden air.
4. Surface Area: The Exposure Factor
The larger the surface area of water exposed to the atmosphere, the faster the evaporation. A wide, shallow puddle evaporates much quicker than a deep, narrow container with the same volume of water. This is because a greater number of water molecules are simultaneously exposed to the atmosphere, increasing the rate of escape. Think of a spilled glass of water versus a lake – the glass evaporates far faster due to its significantly larger surface area-to-volume ratio.
5. Air Pressure: The Weight of the Atmosphere
Air pressure influences evaporation indirectly by affecting the saturation point of the air. Lower air pressure, typically found at higher altitudes, reduces the saturation point, allowing for faster evaporation. Conversely, higher air pressure raises the saturation point, slowing down the process. This is why water evaporates faster at high altitudes than at sea level.
6. Solar Radiation: The Energy Input
Sunlight provides the energy needed for water molecules to overcome the intermolecular forces and transition to the gaseous phase. Strong solar radiation significantly increases evaporation rates, while cloudy conditions reduce it by blocking sunlight. This is why sunny days often lead to quicker drying of clothes compared to overcast days.
Calculating Evaporation Time: The Challenges
While we understand the influential factors, precisely calculating the evaporation time is incredibly complex. There isn't a simple formula to plug in the variables and get an exact answer. This is because the factors interact dynamically and non-linearly. For example, high temperature and low humidity might significantly increase evaporation, but a strong wind might reduce the effect of humidity, making the overall impact difficult to predict precisely.
Numerous empirical formulas and models exist to estimate evaporation rates, each with its limitations and region-specific parameters. These often rely on complex meteorological data, including temperature, humidity, wind speed, solar radiation, and air pressure, which can vary significantly over time and location.
Examples and Real-World Scenarios
Let's illustrate how these factors play out in different scenarios:
-
A puddle on a hot, sunny, windy day: This scenario represents optimal conditions for rapid evaporation. High temperature provides ample energy, low humidity allows for easy vapor uptake, and the wind constantly removes saturated air. This puddle will likely evaporate within hours.
-
A lake in a humid, calm, and shady forest: This scenario represents less favorable conditions. Low temperature, high humidity, absence of wind, and minimal sunlight all contribute to slow evaporation. This lake might take days, weeks, or even months to evaporate significantly.
-
A swimming pool in a dry, desert climate: The intense heat, low humidity, and often strong winds create a perfect storm for rapid evaporation. A swimming pool in this climate would require regular refilling to maintain its water level.
-
A glass of water on a desk in an air-conditioned office: The low temperature, moderate humidity, and absence of wind will result in slow evaporation, potentially taking days or even weeks to fully evaporate.
Beyond Simple Evaporation: Other Factors
The process is further complicated by other factors beyond the primary environmental conditions:
-
Water purity: Impurities in water, such as salts and minerals, can affect surface tension and thus the rate of evaporation.
-
Water depth: While surface area is crucial, water depth also influences evaporation, as deeper water takes longer to heat up and reach the necessary energy levels for evaporation.
-
Substrate: The material beneath the water can also influence evaporation, affecting the heat transfer from the substrate to the water.
Conclusion: A Complex but Vital Process
Determining precisely how long water takes to evaporate is far from a simple task. It depends on a complex interplay of temperature, humidity, wind speed, surface area, air pressure, solar radiation, and other secondary factors. While exact calculation is challenging, understanding these influencing factors provides a nuanced appreciation for this fundamental process that shapes our world, from the weather patterns to the water cycle itself. The key takeaway is that evaporation is not a uniform process; its timescale is incredibly variable and highly context-dependent.
Latest Posts
Latest Posts
-
What Is 1 8 Of An Inch
Mar 10, 2025
-
240 Cm In Inches And Feet
Mar 10, 2025
-
How Big Is A Meter Stick
Mar 10, 2025
-
Least Common Multiple Of 5 And 7
Mar 10, 2025
-
Weight Of One Cubic Meter Of Water
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about How Long Does Water Take To Evaporate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
