How Many Atoms In A Cell
Kalali
Apr 01, 2025 · 6 min read
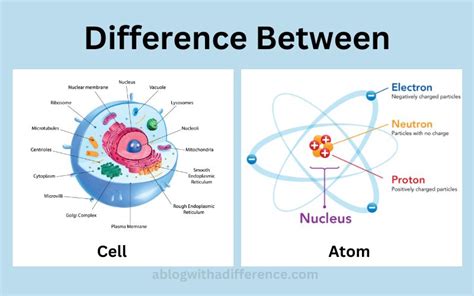
Table of Contents
How Many Atoms Are in a Cell? Unraveling the Cellular Universe
The seemingly simple question, "How many atoms are in a cell?" opens a Pandora's Box of complexity, revealing the intricate and awe-inspiring architecture of life at its most fundamental level. There isn't a single, straightforward answer, as the number varies wildly depending on the type of cell, its size, and its stage of development. However, we can delve into the fascinating science behind estimating this number and appreciate the sheer magnitude of atoms comprising even the smallest living unit.
Understanding the Cellular Landscape: A Diversity of Atoms
Before attempting to quantify the atoms within a cell, it's crucial to acknowledge the sheer diversity of cells. From the minuscule bacteria to the enormously complex human neuron, cell size and composition vary drastically. A bacterial cell, for example, is significantly smaller and simpler than a human cell, leading to a substantial difference in the total atom count.
Furthermore, the composition of a cell isn't uniform. It's a dynamic mixture of various organic and inorganic molecules, each constructed from a unique assortment of atoms. The most prevalent atoms in a cell are:
- Carbon (C): The backbone of organic molecules, forming the foundation of carbohydrates, lipids, proteins, and nucleic acids.
- Hydrogen (H): Abundant in water and organic molecules, playing a critical role in numerous cellular processes.
- Oxygen (O): Essential component of water and a key player in cellular respiration.
- Nitrogen (N): Found in amino acids (building blocks of proteins) and nucleic acids (DNA and RNA).
- Phosphorus (P): Crucial for energy transfer (ATP) and the structure of nucleic acids.
- Sulfur (S): Present in some amino acids and crucial for protein structure and function.
While these six atoms constitute the bulk of a cell's atomic composition, trace amounts of other elements like calcium (Ca), potassium (K), sodium (Na), magnesium (Mg), chlorine (Cl), and iron (Fe) play essential roles in various cellular processes.
Estimating the Atom Count: A Multifaceted Approach
Estimating the number of atoms in a cell requires a multi-pronged approach, combining knowledge of cell size, composition, and the atomic weights of the constituent elements.
1. Cell Size and Volume: A Foundation for Estimation
The first step involves determining the cell's volume. This is typically done using microscopic techniques and mathematical modeling, resulting in a volume expressed in cubic micrometers (µm³). The volume provides a critical estimate of the overall space occupied by the cell's components. Bacterial cells, for instance, might occupy a volume of approximately 1 µm³, while a human cell could range from 100 to 1000 µm³. The vast difference in volume directly impacts the estimated atom count.
2. Cellular Composition: The Molecular Blueprint
Next, we must consider the cellular composition. A cell is primarily composed of water (approximately 70%), along with various macromolecules like proteins, lipids, carbohydrates, and nucleic acids. Knowing the relative abundance of these components is critical in refining the atom count estimation. This involves considering the molecular weight of each component and its contribution to the cell's total mass. For example, proteins, with their rich carbon, hydrogen, oxygen, and nitrogen content, contribute significantly to the overall atom count.
3. Atomic Mass and Avogadro's Number: Bridging the Macro and Micro Worlds
The atomic weight of each element provides the mass of a single atom. Avogadro's number (approximately 6.022 x 10²³) provides the crucial link between the macroscopic world of grams and moles and the microscopic world of atoms and molecules. By calculating the number of moles of each element present in a cell based on its mass and atomic weight, we can then use Avogadro's number to determine the total number of atoms for each element.
4. Summing it All Up: Reaching a Final Estimate
Finally, by adding up the total number of atoms for each element, we arrive at an approximate total atom count for the cell. The final number, however, remains an approximation due to the inherent uncertainties involved in estimating cell volume and composition.
The Magnitude of the Atom Count: A Number Beyond Comprehension
While precise calculations are challenging, estimations suggest that a typical bacterial cell contains on the order of 10¹⁴ atoms, while a human cell might contain 10¹⁶ atoms or more. This massive number is beyond human comprehension. To put it into perspective, imagine trying to count every grain of sand on every beach on Earth; the number of atoms in a single cell dwarfs even that staggering number.
Factors Influencing the Atom Count
Several factors can influence the precise number of atoms in a cell:
- Cell Type: As mentioned earlier, the size and composition of different cell types vary significantly, resulting in differences in atom count.
- Metabolic State: A cell's metabolic activity influences its molecular composition. Cells actively synthesizing proteins or other macromolecules would have a higher atom count compared to dormant cells.
- Environmental Conditions: External factors like nutrient availability and temperature can also affect cellular composition and, consequently, the atom count.
- Cell Cycle Stage: A cell undergoing division will have a different composition and atom count compared to a cell in interphase.
Beyond the Numbers: The Significance of Cellular Complexity
While the sheer number of atoms in a cell is impressive, the true marvel lies in the intricate organization and interaction of these atoms within the cellular structure. The precise arrangement of atoms in molecules, the organization of molecules into organelles, and the coordinated function of these organelles contribute to the cell's remarkable capabilities. Understanding the atomic composition of a cell is fundamental to comprehending the underlying principles of life itself.
Conclusion: A Journey into the Microscopic Universe
The question of how many atoms are in a cell highlights the intricate and awe-inspiring complexity of life at its most fundamental level. Although a precise number is difficult to obtain, the estimations highlight the sheer magnitude of atomic components within even the simplest living unit. This exploration reminds us of the astonishing complexity inherent in even the smallest living entity and underscores the importance of continued research in unraveling the mysteries of cellular biology. The quest to understand the atomic composition of cells is a journey into the microscopic universe, a realm where the fundamental building blocks of life intertwine to create the breathtaking complexity of living organisms. The vast number of atoms working in concert within each cell serves as a testament to the remarkable efficiency and precision of biological systems. Further research into this area will undoubtedly continue to refine our understanding of this microscopic universe and its profound implications for the life sciences.
Latest Posts
Latest Posts
-
7 Liters Is How Many Gallons
Apr 02, 2025
-
Is Baking Soda A Compound Element Or Mixture
Apr 02, 2025
-
What Is 177 Minutes In Hours And Minutes
Apr 02, 2025
-
Cuanto Es El 20 Por Ciento De 20
Apr 02, 2025
-
Is Baking A Cake Physical Or Chemical Change
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms In A Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
