How Many Bonds Does A Carbon Atom Form
Kalali
Mar 21, 2025 · 6 min read
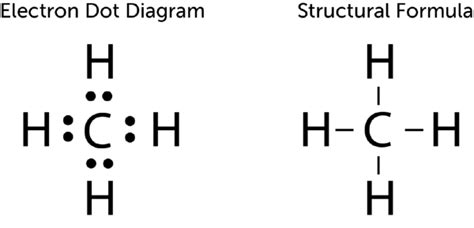
Table of Contents
How Many Bonds Does a Carbon Atom Form? A Deep Dive into Carbon's Bonding Capabilities
Carbon, the backbone of life and a cornerstone of countless materials, possesses a unique bonding versatility that underpins its remarkable properties. Understanding the number of bonds a carbon atom forms is crucial to comprehending its role in organic chemistry, materials science, and beyond. This article delves into the intricacies of carbon bonding, exploring the factors that influence its bonding behavior and the implications of its diverse bonding patterns.
The Octet Rule and Carbon's Valence Electrons
The key to understanding carbon's bonding lies in its electronic structure. Carbon, with an atomic number of 6, possesses six electrons. Two of these electrons reside in the inner shell, while the remaining four occupy the outer, or valence, shell. These four valence electrons are the players in carbon's bonding game. The octet rule, a fundamental principle in chemistry, states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with eight electrons in their outermost shell. For carbon, achieving this stable octet configuration means forming four bonds.
Carbon's Tetravalency: The Foundation of Organic Chemistry
This drive to achieve an octet leads to carbon's tetravalency, its ability to form four covalent bonds. A covalent bond is a chemical bond formed by the sharing of electron pairs between atoms. In the case of carbon, it shares its four valence electrons with other atoms, achieving a full outer shell and forming a stable molecule. This tetravalency is the foundation of organic chemistry, enabling the formation of the incredibly diverse range of organic molecules that make up living organisms and countless synthetic materials.
Types of Bonds Formed by Carbon Atoms
While carbon predominantly forms four bonds, the type of bond can vary, leading to a rich tapestry of molecular structures. These bond types influence the molecule's properties, including its shape, reactivity, and physical characteristics.
Single Bonds: The Simplest Bond
The simplest bond carbon forms is a single bond, involving the sharing of one electron pair between carbon and another atom. Ethane (C₂H₆), for example, features carbon-carbon single bonds and carbon-hydrogen single bonds. Single bonds allow for free rotation around the bond axis, impacting the molecule's flexibility and conformation.
Double Bonds: Sharing Two Electron Pairs
Carbon can also form double bonds, sharing two electron pairs with another atom. Ethene (C₂H₄), also known as ethylene, showcases a carbon-carbon double bond. Double bonds are shorter and stronger than single bonds due to the increased electron density between the atoms. They also restrict rotation around the bond axis, influencing the molecule's rigidity and geometry.
Triple Bonds: The Strongest Bond
Carbon’s bonding versatility extends to triple bonds, where three electron pairs are shared between two carbon atoms. Ethyne (C₂H₂), commonly known as acetylene, exemplifies a carbon-carbon triple bond. Triple bonds are even shorter and stronger than double bonds, imparting considerable rigidity and reactivity to the molecule.
Bonds with Other Atoms: Beyond Carbon-Carbon Bonds
Carbon's remarkable bonding capabilities aren't limited to other carbon atoms. It readily forms bonds with a wide array of other elements, including hydrogen, oxygen, nitrogen, sulfur, and halogens. These bonds are crucial to the structure and function of countless organic molecules, ranging from simple hydrocarbons to complex biomolecules like proteins and DNA.
Factors Influencing Carbon's Bonding Behavior
Several factors influence the types and numbers of bonds a carbon atom forms:
Hybridization: Shaping Carbon's Bonds
The concept of hybridization plays a crucial role in understanding carbon's bonding. Hybridization involves the mixing of atomic orbitals to form hybrid orbitals with different shapes and energies. The most common hybridization states for carbon are:
-
sp³ hybridization: This results in four equivalent sp³ hybrid orbitals, arranged tetrahedrally. This hybridization is typical for single bonds, as seen in alkanes like methane (CH₄).
-
sp² hybridization: This leads to three equivalent sp² hybrid orbitals and one unhybridized p orbital. The sp² orbitals lie in a plane, with the p orbital perpendicular to the plane. This hybridization is characteristic of double bonds, as in alkenes like ethene (C₂H₄).
-
sp hybridization: This produces two equivalent sp hybrid orbitals and two unhybridized p orbitals. The sp orbitals are linear, with the p orbitals perpendicular to each other. This hybridization is found in triple bonds, such as those in alkynes like ethyne (C₂H₂).
The hybridization state dictates the geometry and bonding angles within the molecule, significantly impacting its overall properties.
Electronegativity and Bond Polarity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also plays a role. While carbon is relatively electronegative, its electronegativity varies compared to the atoms it bonds with. Bonds between carbon and highly electronegative atoms like oxygen or nitrogen are polar, resulting in unequal electron sharing. This polarity significantly impacts the molecule's properties, influencing its solubility, reactivity, and interactions with other molecules.
Steric Hindrance: Spatial Considerations
Steric hindrance, the repulsion between atoms or groups of atoms in close proximity, also influences bonding. Bulky groups around a carbon atom can restrict its ability to form certain bonds or influence the preferred conformation of the molecule. This effect is especially important in larger organic molecules and macromolecules.
The Implications of Carbon's Bonding
Carbon's exceptional bonding capabilities have profound implications across various fields:
Organic Chemistry: The Foundation of Life
The tetravalency of carbon is the fundamental reason for the vast diversity of organic molecules. The ability to form long chains, branched structures, and ring systems allows for an almost limitless array of possible compounds, forming the basis of all living organisms and many synthetic materials.
Materials Science: Designing Novel Materials
Carbon's bonding properties are exploited extensively in materials science to design novel materials with specific properties. Diamonds, with their strong carbon-carbon single bonds in a tetrahedral arrangement, are renowned for their hardness, while graphite, with its layered structure and sp² hybridized carbons, is exceptionally soft and a good conductor of electricity. Fullerenes, nanotubes, and graphene, all allotropes of carbon with unique bonding arrangements, represent a frontier of materials research, offering potential applications in electronics, medicine, and energy storage.
Biochemistry: The Building Blocks of Life
The bonding of carbon forms the backbone of biological macromolecules such as proteins, carbohydrates, nucleic acids (DNA and RNA), and lipids. The diverse bonding patterns allow these molecules to adopt complex three-dimensional structures crucial for their biological functions. The specific arrangement and types of bonds in these biomolecules dictate their interactions, influencing their role in cellular processes.
Conclusion: Carbon's Versatile Bonding – A Key to Understanding Our World
The number of bonds a carbon atom forms—predominantly four—is not simply a numerical fact; it is the cornerstone of carbon's exceptional versatility. This tetravalency, coupled with the possibilities of single, double, and triple bonds, hybridization, and interactions with other elements, leads to the vast diversity of organic compounds and materials that shape our world. From the intricate molecules of life to groundbreaking materials of the future, carbon's bonding capabilities continue to fascinate and inspire researchers across countless scientific disciplines. A deeper understanding of carbon's bonding mechanisms is crucial for advancing our knowledge in chemistry, biology, materials science, and many other fields.
Latest Posts
Latest Posts
-
How Many Feet Are In 42 Inches
Mar 28, 2025
-
How Many Acres Is A Square Mile
Mar 28, 2025
-
15 Out Of 50 As A Percentage
Mar 28, 2025
-
How Many Inches In 8 Cm
Mar 28, 2025
-
How To Find Slope Of Vector
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Does A Carbon Atom Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
