How Many Bonds Does Nitrogen Want
Kalali
Mar 31, 2025 · 5 min read
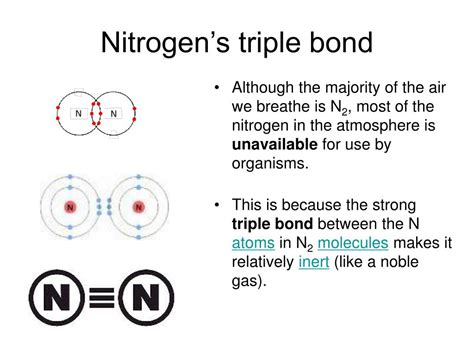
Table of Contents
How Many Bonds Does Nitrogen Want? Understanding Nitrogen's Bonding Behavior
Nitrogen, a crucial element for life as we know it, exhibits a fascinating bonding behavior driven by its electronic configuration. Understanding how many bonds nitrogen "wants" requires delving into its atomic structure and the principles of chemical bonding. This article explores nitrogen's bonding capacity, explaining the factors that influence its bond formation and the diverse range of compounds it forms.
Nitrogen's Electronic Configuration: The Key to Bonding
The answer to "how many bonds does nitrogen want?" lies within its electronic configuration: 1s²2s²2p³. This means nitrogen has five electrons in its outermost shell (valence shell). According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their valence shell, mimicking the electron configuration of noble gases.
Reaching the Octet: Sharing is Caring
Nitrogen achieves a stable octet not by gaining or losing electrons (which would require significant energy), but by sharing electrons through covalent bonding. To reach a full octet, nitrogen needs to share three electrons, resulting in the formation of three covalent bonds.
Types of Bonds Nitrogen Forms
While nitrogen predominantly forms three covalent bonds, the specific types of bonds and the resulting molecular geometries vary depending on the other atoms involved.
1. Single Bonds: The Foundation of Nitrogen's Bonding
The simplest way nitrogen achieves its octet is by forming three single covalent bonds. A classic example is ammonia (NH₃), where nitrogen forms three single bonds with three hydrogen atoms. Each bond involves one electron from nitrogen and one from hydrogen, resulting in a shared electron pair.
2. Double Bonds: Increasing Bond Strength and Stability
Nitrogen can also form double bonds. A prime example is nitrogen dioxide (NO₂), where nitrogen forms one double bond and one single bond with oxygen atoms. The double bond involves the sharing of two pairs of electrons, resulting in a stronger bond compared to a single bond. The unpaired electron on the nitrogen leads to the molecule being a radical.
3. Triple Bonds: The Strongest Bond of All
Nitrogen is unique in its ability to form a triple bond with another nitrogen atom, forming the diatomic molecule nitrogen gas (N₂). This is the most stable form of elemental nitrogen and makes up 78% of the Earth's atmosphere. The triple bond consists of three shared electron pairs, making it exceptionally strong and requiring significant energy to break. This high bond strength is responsible for the relatively inert nature of nitrogen gas.
Factors Influencing Nitrogen's Bonding Behavior
Several factors influence the number and type of bonds nitrogen forms:
1. Electronegativity: Sharing or Partial Transfer?
Nitrogen's electronegativity (3.04 on the Pauling scale) is relatively high, meaning it strongly attracts electrons in a covalent bond. This affects the polarity of the bonds it forms. When bonded to less electronegative atoms (like hydrogen), the bonds are polar covalent, with a slight negative charge on nitrogen. When bonded to more electronegative atoms (like oxygen), the bond polarity shifts, but the overall bonding pattern remains consistent.
2. Steric Hindrance: Space Constraints
The spatial arrangement of atoms around nitrogen also affects bonding. Steric hindrance – the repulsion between electron clouds of atoms – can prevent the formation of multiple bonds if there's not enough space.
3. Resonance: Delocalized Electrons
In some molecules, nitrogen participates in resonance, a phenomenon where electrons are delocalized across multiple bonds. This leads to a more stable molecule with an average bond order (the number of bonds between two atoms, which might not be a whole number). Nitrate ion (NO₃⁻) is a classic example of resonance involving nitrogen.
Exceptions to the Three-Bond Rule: Beyond the Octet
While the octet rule is a useful guideline, it is not without exceptions. In some cases, nitrogen can form more or fewer than three bonds.
1. Hypervalent Nitrogen: Expanding the Octet?
The concept of hypervalent nitrogen, where nitrogen exceeds the octet, is controversial. Although some interpretations suggest the existence of compounds with expanded octets, the evidence is often explained by alternative bonding models that do not involve an expanded valence shell.
2. Coordination Complexes: Nitrogen as a Ligand
Nitrogen can act as a ligand, donating a lone pair of electrons to a central metal atom in coordination complexes. In these complexes, nitrogen contributes a single bond, its lone pair acting as a donor. Ammonia complexes are a prime example.
3. Nitrogen Radicals: Unpaired Electrons
As mentioned earlier in the case of NO₂, nitrogen can exist in radical form with an unpaired electron. These radicals are highly reactive due to their unpaired electron and tend to participate in reactions to pair this electron and achieve a more stable configuration.
The Importance of Understanding Nitrogen's Bonding
Understanding nitrogen's bonding behavior is crucial in various fields:
- Chemistry: Predicting the properties of nitrogen-containing compounds, designing new materials, and understanding chemical reactions.
- Biology: Understanding the role of nitrogen in biological molecules like amino acids, proteins, and nucleic acids. Nitrogen fixation is a critical process for life on Earth.
- Environmental Science: Studying the nitrogen cycle, understanding nitrogen pollution, and developing sustainable nitrogen management strategies.
- Industry: Producing fertilizers, explosives, and other industrial chemicals.
Conclusion: Nitrogen's Versatile Bonding
Nitrogen's "desire" for three bonds is a simplification based on the octet rule and its tendency towards stability. While it predominantly forms three bonds, achieving a stable octet, nitrogen's versatility allows it to participate in diverse bonding arrangements, forming single, double, and triple bonds depending on the specific chemical environment. Understanding the factors influencing its bonding, including electronegativity, steric hindrance, and the potential for resonance, allows us to predict and explain the rich chemistry of nitrogen-containing compounds. Its diverse bonding patterns are fundamental to life and essential across various scientific and industrial applications. The exceptions to the "three-bond rule" further emphasize the complexity and fascinating nature of this essential element's bonding behaviour. Further research continues to refine our understanding of nitrogen's bonding capabilities and their implications.
Latest Posts
Latest Posts
-
What Is 3 10 As A Decimal
Apr 02, 2025
-
0 25 Mole Of Mg Contains How Many Atoms
Apr 02, 2025
-
5 Lb Is How Many Oz
Apr 02, 2025
-
Cuanto Es 100 Mililitros En Onzas
Apr 02, 2025
-
10 Oz Is How Many Cups
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Does Nitrogen Want . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
