How Many Carbon Atoms Are Contained In 2.8g Of C2h4
Kalali
Mar 23, 2025 · 5 min read
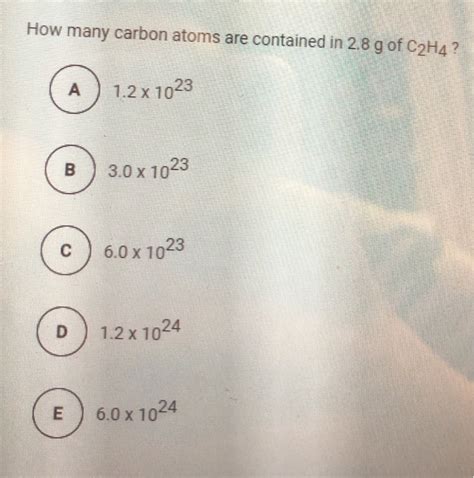
Table of Contents
How Many Carbon Atoms are Contained in 2.8g of C₂H₄? A Comprehensive Guide
Determining the number of carbon atoms in a given mass of a compound requires a systematic approach combining fundamental chemistry principles with Avogadro's number. This article will guide you through the step-by-step process of calculating the number of carbon atoms in 2.8g of ethylene (C₂H₄), explaining each concept thoroughly. We will also explore related concepts to deepen your understanding of stoichiometry and Avogadro's constant.
Understanding the Basics: Moles, Molar Mass, and Avogadro's Number
Before diving into the calculation, let's refresh our understanding of crucial concepts:
1. Moles:
The mole (mol) is the fundamental unit of measurement in chemistry. It represents a specific number of entities (atoms, molecules, ions, etc.). One mole of any substance contains Avogadro's number of particles.
2. Molar Mass:
The molar mass (M) of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's essentially the atomic weight (or molecular weight for compounds) expressed in grams. For example, the molar mass of carbon (C) is approximately 12.01 g/mol, and the molar mass of hydrogen (H) is approximately 1.01 g/mol.
3. Avogadro's Number:
Avogadro's number (N<sub>A</sub>) is a fundamental constant representing the number of entities in one mole of a substance. Its value is approximately 6.022 x 10<sup>23</sup>. This means one mole of any substance contains 6.022 x 10<sup>23</sup> atoms, molecules, ions, or other specified entities.
Calculating the Number of Carbon Atoms in 2.8g of C₂H₄
Now, let's break down the calculation into clear, manageable steps:
Step 1: Determine the molar mass of C₂H₄
Ethylene (C₂H₄) consists of two carbon atoms and four hydrogen atoms. To calculate its molar mass, we sum the molar masses of its constituent atoms:
- Molar mass of C = 12.01 g/mol
- Molar mass of H = 1.01 g/mol
Molar mass of C₂H₄ = (2 × 12.01 g/mol) + (4 × 1.01 g/mol) = 24.02 g/mol + 4.04 g/mol = 28.06 g/mol
Step 2: Calculate the number of moles of C₂H₄
We can use the following formula to determine the number of moles (n) of C₂H₄:
n = mass / molar mass
n = 2.8 g / 28.06 g/mol ≈ 0.0998 moles of C₂H₄
Step 3: Determine the number of carbon atoms
One molecule of C₂H₄ contains two carbon atoms. Therefore, one mole of C₂H₄ contains 2 x N<sub>A</sub> carbon atoms. To find the total number of carbon atoms in 0.0998 moles of C₂H₄, we perform the following calculation:
Number of carbon atoms = (number of moles of C₂H₄) × (number of carbon atoms per molecule) × (Avogadro's number)
Number of carbon atoms = 0.0998 mol × 2 atoms/molecule × 6.022 x 10<sup>23</sup> molecules/mol
Number of carbon atoms ≈ 1.20 x 10<sup>23</sup> carbon atoms
Expanding the Understanding: Further Exploration of Related Concepts
This calculation provides a fundamental understanding of stoichiometry. Let's delve deeper into related concepts to enhance your grasp of this crucial chemical principle:
1. Percentage Composition:
Understanding the percentage composition of a compound is essential in various chemical analyses. For C₂H₄:
- Percentage of Carbon: [(2 × 12.01 g/mol) / 28.06 g/mol] × 100% ≈ 85.7%
- Percentage of Hydrogen: [(4 × 1.01 g/mol) / 28.06 g/mol] × 100% ≈ 14.3%
This shows that ethylene is primarily composed of carbon.
2. Empirical and Molecular Formulas:
The formula C₂H₄ is the molecular formula, representing the actual number of atoms in one molecule. The empirical formula represents the simplest whole-number ratio of atoms in a compound. In this case, the empirical formula is also CH₂ because the ratio of carbon to hydrogen is 1:2. Determining the empirical formula from experimental data is a crucial step in identifying unknown compounds.
3. Stoichiometric Calculations in Chemical Reactions:
The principles discussed here are fundamental to stoichiometric calculations involving chemical reactions. For instance, if C₂H₄ undergoes combustion, we can use these principles to calculate the amount of CO₂ and H₂O produced based on the balanced chemical equation.
4. Significance of Avogadro's Number:
Avogadro's number is a cornerstone of chemistry, bridging the macroscopic world (grams) with the microscopic world (atoms and molecules). It allows us to connect measurable quantities like mass with the actual number of particles involved in a chemical system.
5. Sources of Error:
The calculation presented assumes perfect conditions and uses approximate molar masses. In a real-world scenario, experimental errors might lead to slight variations in the final result. These errors can arise from inaccuracies in weighing the sample, impurities in the ethylene sample, or limitations in the measuring instruments.
Conclusion: A Precise and Detailed Calculation
This detailed explanation comprehensively illustrates how to calculate the number of carbon atoms in 2.8g of C₂H₄. By understanding moles, molar mass, Avogadro's number, and related concepts, you can confidently tackle similar stoichiometric problems. Remember that the key is to systematically proceed through the steps, ensuring a clear understanding of each calculation. This approach not only yields the correct numerical answer but also cultivates a deeper understanding of fundamental chemical principles. Furthermore, grasping these concepts is crucial for tackling more complex chemical calculations and analyses.
Latest Posts
Latest Posts
-
85 Cm Is How Many Inches
Mar 24, 2025
-
What Is 30 Percent Of 400
Mar 24, 2025
-
How Long Is 25 Cm In Inches
Mar 24, 2025
-
How Long Is 22 Cm In Inches
Mar 24, 2025
-
How Many Different Sequences Of Eight Bases Can You Make
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Many Carbon Atoms Are Contained In 2.8g Of C2h4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
