How Many Carbons Are In The Planar Double Bond System
Kalali
Mar 31, 2025 · 5 min read
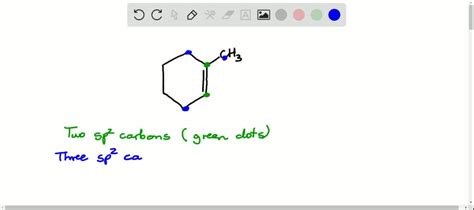
Table of Contents
Delving into Planar Double Bond Systems: A Comprehensive Look at Carbon Count and Conjugation
The question of how many carbons are in a planar double bond system isn't straightforward. It doesn't have a single numerical answer, as the number of carbons depends entirely on the specific molecule in question. Instead, understanding this question requires a deeper dive into the concept of conjugated systems, delocalized pi electrons, and the structural requirements for planarity. This exploration will cover the fundamental principles, illustrative examples, and nuances of these fascinating molecular structures.
Understanding Conjugation and Planarity
The key to answering the implicit question lies in understanding conjugation. Conjugation refers to a system of connected p-orbitals with alternating single and multiple bonds. This arrangement allows for the delocalization of pi electrons across the entire conjugated system. Crucially, for effective conjugation and resulting planarity, these p-orbitals must be able to overlap effectively. This necessitates a specific geometry: planarity. The atoms involved in the conjugated system must lie in the same plane to maximize p-orbital overlap and achieve effective delocalization.
Why Planarity is Crucial:
Imagine the p-orbitals as parallel, slightly sideways overlapping clouds of electrons. If the atoms are not coplanar, the overlap is significantly reduced or even nonexistent. This diminishes the delocalization effect, and the molecule loses many of the unique properties associated with conjugated systems. These properties include:
- Increased stability: Delocalization lowers the overall energy of the system.
- Altered reactivity: Conjugated systems often exhibit unique reactivity patterns compared to their non-conjugated counterparts.
- Specific spectral properties: The delocalized pi electrons often lead to characteristic UV-Vis absorption.
Counting Carbons in Different Conjugated Systems
The number of carbons involved directly dictates the extent of the delocalization and the associated properties. Let's explore several examples:
1. Simple Conjugated Dienes:
The simplest example involves a conjugated diene, such as 1,3-butadiene (CH₂=CH-CH=CH₂). This molecule has four carbons involved in the conjugated system. Each carbon atom possesses a p-orbital participating in the delocalized pi electron cloud. The molecule is planar to allow maximum overlap of these p-orbitals.
2. Longer Conjugated Chains:
As the chain length increases, so does the number of carbons involved in conjugation. Consider a conjugated triene, like 1,3,5-hexatriene (CH₂=CH-CH=CH-CH=CH₂). This molecule contains six carbons in the conjugated system. The increased conjugation leads to a greater degree of delocalization and enhanced stability.
3. Aromatic Compounds:
Aromatic compounds represent a special class of conjugated systems characterized by a cyclic structure fulfilling Huckel's rule (4n+2 pi electrons, where n is an integer). Benzene (C₆H₆) is the archetypal example. All six carbon atoms in benzene are involved in the conjugated system, forming a highly stable planar ring. This delocalization significantly contributes to benzene's exceptional stability and unique reactivity.
4. Extended Conjugation in Polyenes:
Conjugated polyenes, such as beta-carotene, contain extremely long chains of conjugated double bonds. Beta-carotene, a pigment found in many plants, has a conjugated system extending across 11 carbon atoms. The extensive conjugation is responsible for its vibrant orange color and its role in photosynthesis. The large number of conjugated carbons leads to a significant delocalization of electrons.
5. Heterocyclic Conjugated Systems:
Conjugation isn't limited to carbon-carbon double bonds. Heterocyclic aromatic compounds, such as pyridine and pyrrole, incorporate heteroatoms (atoms other than carbon) into the conjugated ring system. Pyridine, for example, features a nitrogen atom within the six-membered aromatic ring. Despite the heteroatom, all six atoms (five carbons and one nitrogen) participate in the delocalized pi electron system, maintaining planarity.
Factors Affecting Planarity and Conjugation
While the examples above illustrate the general principle, various factors can influence the extent of conjugation and the planarity of the system:
-
Steric Hindrance: Bulky substituents attached to the conjugated system may cause steric clashes, forcing the molecule out of planarity and reducing the effectiveness of conjugation.
-
Ring Strain: In cyclic conjugated systems, ring strain can distort the geometry, hindering optimal p-orbital overlap. Highly strained rings may deviate significantly from planarity.
-
Cross-Conjugation: In certain molecules, the double bonds are not directly connected but are linked through a single bond. This cross-conjugation can lead to less effective delocalization compared to linear conjugation.
-
Conformational Isomerism: Some molecules can adopt different conformations that affect the degree of conjugation. A conformation that allows for maximum p-orbital overlap would show more effective conjugation.
Beyond Simple Counting: Understanding the Implications of Conjugation
The number of carbons in a planar double bond system is crucial, but it's not the sole determinant of the molecule's properties. The arrangement of those carbons, the presence of heteroatoms, and the overall molecular structure all contribute to the molecule's chemical behavior and physical characteristics.
Spectral Properties: The extent of conjugation directly impacts the wavelength of light absorbed by the molecule. Longer conjugated systems absorb light at longer wavelengths, resulting in a shift towards the visible region of the electromagnetic spectrum. This explains the colors observed in many conjugated molecules.
Reactivity: Conjugated systems often exhibit unique reactivity patterns compared to non-conjugated systems. Electrophilic aromatic substitution, for instance, is a characteristic reaction of aromatic compounds due to the presence of the delocalized pi electron cloud.
Stability: Delocalization of electrons increases the overall stability of the conjugated system, making it less prone to chemical reactions compared to isolated double bonds.
Conclusion
The question of "how many carbons are in a planar double bond system?" doesn't have a simple numerical answer. The number of carbons involved varies greatly depending on the specific molecule. Understanding conjugation, planarity, and their implications are vital for comprehending the unique properties and reactivity of these systems. From simple dienes to complex aromatic compounds and extended polyenes, the interplay between the number of conjugated carbons, their arrangement, and other structural features shapes the molecule's behavior and properties. This comprehensive understanding is crucial in various fields, including organic chemistry, materials science, and biochemistry. Future research continues to uncover the fascinating complexities and potential applications of these molecules.
Latest Posts
Latest Posts
-
30 To The Power Of 2
Apr 02, 2025
-
What Is 400 Fahrenheit In Centigrade
Apr 02, 2025
-
50 Oz Is How Many Liters
Apr 02, 2025
-
How Many Feet Is 109 Inches
Apr 02, 2025
-
What Is In Both Eukaryotic And Prokaryotic Cells
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Carbons Are In The Planar Double Bond System . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
