How Many Electrons Are In Chlorine
Kalali
Mar 10, 2025 · 5 min read
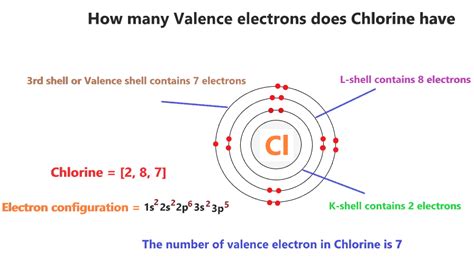
Table of Contents
How Many Electrons Are in Chlorine? A Deep Dive into Atomic Structure
Chlorine, a vital element with a pungent smell and a pale green-yellow gas appearance, plays a crucial role in various aspects of our lives, from water purification to industrial processes. Understanding its atomic structure, especially the number of electrons it possesses, is fundamental to comprehending its chemical behavior and reactivity. This comprehensive guide will not only answer the question "How many electrons are in chlorine?" but will also delve into the broader context of atomic structure, electron configuration, and the significance of electron count in determining an element's properties.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in chlorine, let's establish a foundational understanding of atomic structure. Every atom consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines an element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom, ensuring a balanced charge.
Chlorine's Atomic Number and Electron Count
Chlorine's atomic number is 17. This crucial number indicates that a neutral chlorine atom contains 17 protons in its nucleus. Since a neutral atom has an equal number of protons and electrons, a neutral chlorine atom also has 17 electrons.
This seemingly simple fact holds profound implications for chlorine's chemical behavior and its ability to form bonds with other elements. The arrangement of these 17 electrons in different energy levels determines its reactivity and the types of compounds it can form.
Electron Configuration: Unveiling the Arrangement of Electrons
The electrons in an atom are not randomly distributed. They occupy specific energy levels or shells, each capable of holding a maximum number of electrons. These energy levels are arranged in increasing order of energy, with electrons filling the lower energy levels first. This arrangement is described by the element's electron configuration.
Chlorine's electron configuration is 1s²2s²2p⁶3s²3p⁵. Let's break this down:
- 1s²: The first energy level (n=1) contains one subshell, designated as 's', which can hold a maximum of two electrons. Chlorine has two electrons in this subshell.
- 2s²: The second energy level (n=2) also contains an 's' subshell, holding another two electrons.
- 2p⁶: The second energy level also contains three 'p' subshells, each capable of holding two electrons, for a total of six electrons in the 2p subshell.
- 3s²: The third energy level (n=3) begins with an 's' subshell, holding two electrons.
- 3p⁵: Finally, the third energy level contains three 'p' subshells, but only five of these six available spaces are occupied by electrons in chlorine.
This incomplete 3p subshell is the key to understanding chlorine's reactivity. Atoms strive for a stable electron configuration, often resembling that of the noble gases (Group 18 elements) which have full outer electron shells. Chlorine's tendency to gain one electron to achieve a full 3p subshell (and thus a stable octet of electrons in its outermost shell) explains its high electronegativity and its ability to readily form ionic bonds.
Isotopes of Chlorine and Electron Count
While the number of protons defines an element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Chlorine has two naturally occurring isotopes:
- Chlorine-35 (³⁵Cl): This isotope constitutes approximately 75% of naturally occurring chlorine and contains 17 protons and 18 neutrons. It still has 17 electrons, maintaining electrical neutrality.
- Chlorine-37 (³⁷Cl): This isotope makes up roughly 25% of naturally occurring chlorine and contains 17 protons and 20 neutrons. Again, it has 17 electrons, maintaining neutrality.
The different number of neutrons affects the mass of the isotope but doesn't alter the number of electrons in a neutral atom. Therefore, regardless of the isotope, a neutral chlorine atom will always possess 17 electrons.
The Significance of Chlorine's Electron Count in Chemical Reactions
Chlorine's 17 electrons, specifically its seven valence electrons (electrons in the outermost shell), dictate its chemical behavior. This incomplete outer shell makes chlorine highly reactive. It readily gains an electron to achieve a stable octet, forming a negatively charged chloride ion (Cl⁻). This electron gain is a fundamental characteristic of chlorine's participation in chemical reactions.
This strong tendency to gain an electron explains:
- Its ability to form ionic bonds: Chlorine readily reacts with metals, accepting an electron from the metal to form stable ionic compounds like sodium chloride (NaCl, common table salt).
- Its role in covalent bonding: Chlorine can also share electrons with other non-metals to form covalent bonds, resulting in molecules like hydrogen chloride (HCl), a strong acid.
- Its use as an oxidizing agent: Chlorine's ability to accept electrons makes it a strong oxidizing agent, meaning it can readily accept electrons from other substances, causing them to be oxidized. This property is crucial in many industrial and environmental applications, such as water purification.
Chlorine's Importance in Various Applications
The unique properties arising from its atomic structure, particularly its 17 electrons, make chlorine indispensable in various applications:
- Water purification: Chlorine effectively disinfects water by killing harmful bacteria and viruses, safeguarding public health.
- Industrial processes: It plays a vital role in the production of various chemicals, including plastics, solvents, and pesticides.
- Medical applications: Chlorine compounds find use in disinfectants and pharmaceuticals.
- Bleaching: Chlorine is a powerful bleaching agent, used in the paper and textile industries.
Conclusion: The Significance of 17 Electrons
In conclusion, a neutral chlorine atom always contains 17 electrons. This seemingly simple fact is central to understanding its chemical behavior, reactivity, and its widespread applications. The arrangement of these electrons, specifically the seven valence electrons, drives its ability to form bonds, act as an oxidizing agent, and play a significant role in numerous industrial and environmental processes. Understanding the relationship between electron configuration, atomic structure, and chemical properties provides a deeper appreciation of chlorine's importance in our world. The number 17, therefore, is much more than just a number; it is the key to understanding the multifaceted role of chlorine in our daily lives and beyond.
Latest Posts
Latest Posts
-
What Percent Of 50 Is 9
May 09, 2025
-
31 Out Of 40 Is What Percentage
May 09, 2025
-
Properties Of Rhombus Rectangle And Square
May 09, 2025
-
How Many Hours Is 133 Minutes
May 09, 2025
-
Cuantos Son 72 Grados Fahrenheit En Centigrados
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.