How Many Electrons Can An Orbital Hold
Kalali
Jun 09, 2025 · 3 min read
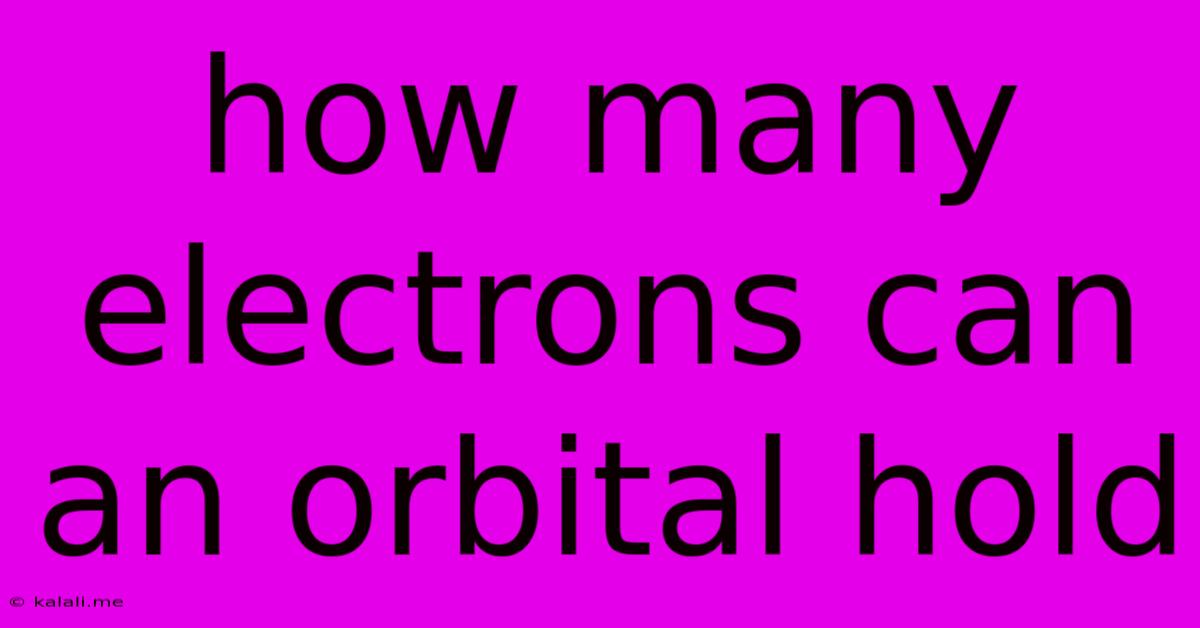
Table of Contents
How Many Electrons Can an Orbital Hold? A Deep Dive into Atomic Structure
Understanding how many electrons an orbital can hold is fundamental to grasping the basics of chemistry and atomic structure. This article will explore the answer to this question, delving into the concepts of electron shells, subshells, and the Pauli Exclusion Principle. We'll also touch upon the implications of this limitation on the periodic table and chemical bonding.
The simple answer is: an orbital can hold a maximum of two electrons. However, understanding why this is the case requires a closer look at the quantum mechanical model of the atom.
Electron Shells, Subshells, and Orbitals: A Quick Recap
Electrons within an atom are arranged in energy levels called electron shells. Each shell can accommodate a specific number of electrons. These shells are further divided into subshells, denoted by the letters s, p, d, and f. Finally, within each subshell are orbitals, which represent regions of space where there's a high probability of finding an electron.
- s subshell: Contains one spherical orbital.
- p subshell: Contains three dumbbell-shaped orbitals.
- d subshell: Contains five orbitals with more complex shapes.
- f subshell: Contains seven orbitals with even more intricate shapes.
The Pauli Exclusion Principle: The Key to Electron Capacity
The key to understanding the maximum number of electrons per orbital lies in the Pauli Exclusion Principle. This principle states that no two electrons within an atom can have the same set of four quantum numbers. These quantum numbers describe the electron's state:
- Principal quantum number (n): Describes the energy level or shell.
- Azimuthal quantum number (l): Describes the subshell (s, p, d, f).
- Magnetic quantum number (ml): Describes the specific orbital within a subshell.
- Spin quantum number (ms): Describes the intrinsic angular momentum of the electron, often represented as +1/2 (spin up) or -1/2 (spin down).
Since there are only two possible values for the spin quantum number (ms), an orbital, defined by a unique combination of n, l, and ml, can only hold a maximum of two electrons – one with spin up and one with spin down.
Implications on the Periodic Table and Chemical Bonding
The limitation of two electrons per orbital directly influences the arrangement of elements in the periodic table. The periodic table's structure reflects the filling of electron shells and subshells, with each column representing elements with similar outer electron configurations. This outer electron configuration, particularly the number of electrons in the outermost shell (valence electrons), dictates an element's chemical properties and reactivity. The way atoms share or transfer electrons to achieve stable electron configurations (often a full outer shell) forms the basis of chemical bonding.
Beyond the Basics: Exceptions and Complexities
While the "two electrons per orbital" rule is a fundamental concept, it's essential to acknowledge that exceptions exist, particularly in the realm of transition metals and other complex atoms. However, for a basic understanding of atomic structure, this rule provides a solid foundation.
In conclusion, the maximum number of electrons an orbital can hold is two, dictated by the Pauli Exclusion Principle. This seemingly simple rule underpins much of our understanding of atomic structure, chemical bonding, and the organization of the periodic table, shaping the behavior of matter as we know it. Understanding this principle is crucial for further exploration into the fascinating world of chemistry and physics.
Latest Posts
Latest Posts
-
Why Does My Bed Creak When I Move
Jun 09, 2025
-
When Atc Calls For Gaurd What Does That Mean
Jun 09, 2025
-
Chacos Toe Strap Or No Toe Strap
Jun 09, 2025
-
How To Check A Fuel Pressure Regulator
Jun 09, 2025
-
I Am The Lord Thy God
Jun 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can An Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.