How Many Electrons Can D Orbital Hold
Kalali
Mar 10, 2025 · 6 min read
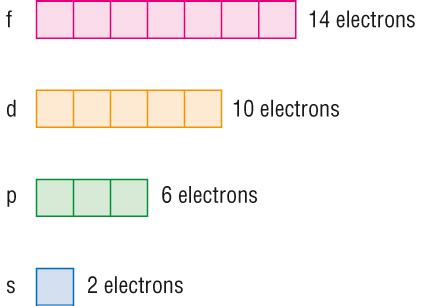
Table of Contents
How Many Electrons Can a d Orbital Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to chemistry. A key component of this understanding involves grasping the capacity of different atomic orbitals, particularly the d orbitals. This article will delve into the intricacies of d orbitals, explaining not only how many electrons they can hold but also the underlying principles of quantum mechanics that govern their behavior.
Understanding Atomic Orbitals
Before we focus on d orbitals specifically, let's review the basics of atomic orbitals. Atomic orbitals are regions of space around an atomic nucleus where there's a high probability of finding an electron. These orbitals are described by quantum numbers, which provide a mathematical description of the electron's state. These numbers are:
-
Principal Quantum Number (n): Determines the energy level and size of the orbital. n can be any positive integer (1, 2, 3...). Higher n values mean higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): Determines the shape of the orbital and its angular momentum. l can be any integer from 0 to n - 1. Different values of l correspond to different orbital types:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): Specifies the orientation of the orbital in space. ml can take on any integer value from -l to +l, including 0. This means:
- s orbitals (l=0) have 1 orbital (ml=0)
- p orbitals (l=1) have 3 orbitals (ml=-1, 0, +1)
- d orbitals (l=2) have 5 orbitals (ml=-2, -1, 0, +1, +2)
- f orbitals (l=3) have 7 orbitals (ml=-3, -2, -1, 0, +1, +2, +3)
-
Spin Quantum Number (ms): Describes the intrinsic angular momentum of the electron, often visualized as "spin up" (+1/2) or "spin down" (-1/2). This is independent of the other quantum numbers.
The d Orbitals: Shape and Spatial Orientation
The d orbitals, characterized by l = 2, have a more complex shape than s or p orbitals. They possess five distinct spatial orientations, each corresponding to a specific value of the magnetic quantum number (ml). These orientations are often represented visually, though understanding the precise mathematical functions describing them requires advanced knowledge of quantum mechanics. The five d orbitals are usually labeled as:
- d<sub>xy</sub>: Electron density concentrated in the xy-plane, between the x and y axes.
- d<sub>xz</sub>: Electron density concentrated in the xz-plane, between the x and y axes.
- d<sub>yz</sub>: Electron density concentrated in the yz-plane, between the y and z axes.
- d<sub>x²−y²</sub>: Electron density concentrated along the x and y axes, with a nodal plane at 45 degrees.
- d<sub>z²</sub>: Electron density concentrated along the z-axis, with a toroidal shape in the xy-plane.
These complex shapes are crucial in understanding the chemical bonding properties of transition metal atoms and ions, where d orbitals play a pivotal role.
The Electron Capacity of a d Orbital
Each individual d orbital can hold a maximum of two electrons, according to the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers. Since each electron within a d orbital must have different spin quantum numbers (either +1/2 or -1/2), only two electrons can occupy a single d orbital.
The Electron Capacity of the d Subshell
The d subshell, encompassing all five d orbitals, can therefore hold a maximum of ten electrons. This is because each of the five d orbitals can accommodate two electrons, resulting in a total capacity of 2 electrons/orbital * 5 orbitals = 10 electrons.
Filling d Orbitals: Hund's Rule and Electron Configuration
When filling d orbitals with electrons, two important rules must be considered:
-
Aufbau Principle: Electrons fill orbitals in order of increasing energy. Generally, the energy order is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... (Note that there can be exceptions to this order, particularly in transition metals).
-
Hund's Rule: Within a subshell (like the d subshell), electrons will individually occupy each orbital before doubling up in any one orbital. This is because electrons repel each other and prefer to be in separate orbitals with parallel spins (maximizing total spin).
For example, consider Chromium (Cr), which has an atomic number of 24. Following the Aufbau and Hund's rules, its electron configuration is [Ar] 3d<sup>5</sup> 4s<sup>1</sup>, not [Ar] 3d<sup>4</sup> 4s<sup>2</sup> as might be initially expected based on a simplistic energy level understanding. This exception highlights the subtle complexities of electron configurations.
Importance of d Orbitals in Chemistry
The d orbitals play a crucial role in various chemical phenomena:
-
Transition Metal Chemistry: Transition metals are characterized by partially filled d orbitals. The variable oxidation states and the formation of coloured complexes are direct consequences of the d electrons participating in chemical bonding.
-
Catalysis: Many transition metal compounds act as catalysts, facilitating chemical reactions. The ability of d orbitals to participate in redox reactions is key to their catalytic activity.
-
Coordination Compounds: Transition metals form coordination compounds with ligands, which are molecules or ions that donate electron pairs to the metal. These interactions involve the d orbitals of the metal ion.
-
Spectroscopy: The electronic transitions between d orbitals are responsible for the characteristic colors observed in many transition metal complexes. Studying these transitions allows for the determination of the electronic structure and geometry of the complexes.
-
Magnetic Properties: The presence of unpaired electrons in d orbitals leads to the paramagnetic behaviour of many transition metal compounds.
Beyond the Basics: Advanced Concepts
The description of d orbitals presented here is a simplified model. In reality, the interactions between electrons and the nucleus are far more complex. Advanced quantum mechanical calculations are required to obtain a more accurate representation of electron distribution. Furthermore, the concept of orbital hybridization, where atomic orbitals mix to form new hybrid orbitals, is crucial for understanding the bonding in many molecules and ions.
Furthermore, relativistic effects, especially important for heavier elements, can influence the energy levels and spatial distribution of d orbitals. These effects become more pronounced as the atomic number increases, influencing the chemical properties of the heavier transition metals.
Conclusion
In conclusion, a d orbital can hold a maximum of two electrons due to the Pauli Exclusion Principle. The d subshell, containing five d orbitals, can therefore hold a maximum of ten electrons. However, the actual filling of d orbitals follows specific rules, and exceptions are observed in some transition metal atoms due to the complexities of electron-electron interactions and orbital energy levels. Understanding the capacity and behavior of d orbitals is critical for comprehending the diverse and fascinating chemistry of transition metal compounds and their applications in various fields. Their influence extends to catalysis, spectroscopy, magnetic properties, and coordination chemistry, underscoring their fundamental importance in the world of atomic structure and chemical bonding.
Latest Posts
Latest Posts
-
Least Common Multiple Of 4 And 8
Mar 11, 2025
-
Are Covalent Compounds Soluble In Water
Mar 11, 2025
-
2 To The Power Of 64
Mar 11, 2025
-
How Much Is A Gallon Water
Mar 11, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can D Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
