How Many Electrons Can The D Sublevel Hold
Kalali
Mar 15, 2025 · 7 min read
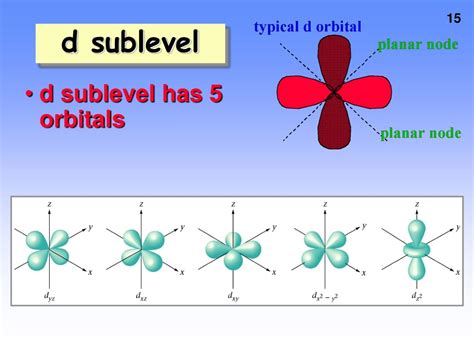
Table of Contents
How Many Electrons Can the d Sublevel Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to chemistry and physics. A key aspect of this understanding lies in grasping the capacity of different electron sublevels. This article will delve deep into the question: how many electrons can the d sublevel hold? We'll explore the underlying principles of quantum mechanics, the structure of atoms, and the implications of the d sublevel's electron capacity for chemical properties and periodic trends.
Understanding Electron Shells, Subshells, and Orbitals
Before tackling the d sublevel specifically, let's review the basic structure of an atom. Electrons reside in energy levels, often called shells, surrounding the nucleus. Each shell is further divided into subshells, designated by the letters s, p, d, and f. These subshells represent different regions of space where electrons are most likely to be found. Within each subshell are orbitals, which are specific three-dimensional regions that can hold a maximum of two electrons, according to the Pauli Exclusion Principle. This principle states that no two electrons in an atom can have the same set of four quantum numbers.
The Quantum Numbers: A Key to Understanding Electron Configuration
The behavior of electrons within an atom is governed by four quantum numbers:
-
Principal Quantum Number (n): This determines the energy level or shell (n = 1, 2, 3,...). Higher values of n indicate higher energy levels and greater distance from the nucleus.
-
Azimuthal Quantum Number (l): This specifies the subshell, ranging from 0 to n - 1. l = 0 corresponds to the s subshell, l = 1 to the p subshell, l = 2 to the d subshell, and l = 3 to the f subshell.
-
Magnetic Quantum Number (ml): This describes the orientation of the orbital in space. It ranges from -l to +l, including 0. For example, the p subshell (l = 1) has three orbitals (ml = -1, 0, +1).
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum of an electron, with values of +1/2 or -1/2, often represented as "spin up" and "spin down."
The d Sublevel: Shape, Orbitals, and Electron Capacity
The d subshell (l = 2) is characterized by its five orbitals. The magnetic quantum number (ml) for the d subshell can take values of -2, -1, 0, +1, +2. Therefore, the d subshell has five orbitals, each capable of holding two electrons (one spin up and one spin down).
Visualizing the d Orbitals
While visualizing the precise shapes of d orbitals can be challenging, it's important to understand they are more complex than the spherical s orbitals and dumbbell-shaped p orbitals. They exhibit more intricate shapes with multiple lobes and nodal planes. The five d orbitals are often labeled as d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>, d<sub>x²-y²</sub>, and d<sub>z²</sub>, reflecting their orientation in three-dimensional space.
Calculating the Maximum Number of Electrons in the d Sublevel
Since each of the five d orbitals can hold a maximum of two electrons, the total number of electrons that the d sublevel can hold is 5 orbitals × 2 electrons/orbital = 10 electrons.
The Significance of the d Sublevel in Chemistry
The d sublevel plays a crucial role in the chemistry of transition metals. These elements are characterized by partially filled d subshells in their atoms or ions. The presence of these d electrons leads to several important chemical properties:
-
Variable Oxidation States: Transition metals can exhibit multiple oxidation states because the d electrons can participate in bonding in various ways. This versatility is crucial for their use in catalysis and other applications.
-
Colored Compounds: The d electrons can absorb specific wavelengths of light, leading to the characteristic colors of many transition metal compounds. This is a consequence of electronic transitions between different d orbitals.
-
Paramagnetism: The presence of unpaired electrons in the d subshell often results in paramagnetism, the tendency of a substance to be attracted to a magnetic field.
-
Catalytic Activity: The ability of transition metals to readily gain and lose electrons, coupled with their variable oxidation states, makes them excellent catalysts in many chemical reactions.
Filling the d Sublevel: Hund's Rule and Aufbau Principle
When filling electron subshells, two principles guide the process:
-
Aufbau Principle: Electrons fill orbitals starting with the lowest energy levels first. Generally, the filling order follows the sequence 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p. However, exceptions can occur due to subtle energy level variations.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital with parallel spins before pairing up. This maximizes the total spin and stabilizes the electron configuration.
Understanding these rules is crucial for accurately predicting the electron configurations of atoms and ions.
Examples: Electron Configurations with Filled and Partially Filled d Sublevels
Let's look at a few examples:
-
Chromium (Cr): Its atomic number is 24. You might expect the electron configuration to be [Ar] 3d<sup>4</sup> 4s<sup>2</sup>, but due to the stability of a half-filled d subshell, the actual configuration is [Ar] 3d<sup>5</sup> 4s<sup>1</sup>.
-
Copper (Cu): Similarly, copper (atomic number 29) has an electron configuration of [Ar] 3d<sup>10</sup> 4s<sup>1</sup> rather than [Ar] 3d<sup>9</sup> 4s<sup>2</sup>, favoring a completely filled d subshell.
-
Zinc (Zn): Zinc (atomic number 30) has a completely filled d subshell: [Ar] 3d<sup>10</sup> 4s<sup>2</sup>.
These exceptions highlight the complexities of electron filling and the importance of considering electron-electron repulsions and the stability of half-filled and fully filled subshells.
The d Sublevel in Coordination Chemistry
The d sublevel plays a pivotal role in coordination chemistry, the study of metal complexes. In these complexes, a central metal ion is surrounded by ligands (molecules or ions) that donate electron pairs to the metal. The interaction between the d orbitals of the metal and the orbitals of the ligands significantly affects the properties of the complex, including its color, magnetic properties, and reactivity. Crystal field theory and ligand field theory provide frameworks for understanding these interactions and predicting the properties of coordination compounds.
Applications Utilizing the Properties of the d Sublevel
The unique properties stemming from the d sublevel have led to numerous applications across various fields:
-
Catalysis: Transition metals, with their variable oxidation states and readily available d electrons, are extensively used as catalysts in industrial processes, such as the Haber-Bosch process for ammonia synthesis and various petroleum refining processes.
-
Pigments and Dyes: The ability of transition metal compounds to absorb and reflect specific wavelengths of light makes them valuable pigments and dyes in paints, textiles, and other materials.
-
Magnetism: Materials with partially filled d orbitals often exhibit magnetic properties, making them crucial components in magnets and magnetic storage devices.
-
Biochemistry: Transition metals play essential roles in many biological systems, often acting as cofactors in enzymes. For example, iron in hemoglobin is crucial for oxygen transport.
Conclusion
The d sublevel, with its capacity to hold up to 10 electrons, is a crucial aspect of atomic structure. Its influence extends significantly to the chemical and physical properties of transition metals, impacting a broad range of applications from catalysis and pigments to magnetic materials and biological systems. Understanding the intricacies of electron configuration, including the rules governing the filling of the d sublevel, is fundamental to comprehending the diverse behavior of matter at the atomic and molecular level. Further exploration into quantum mechanics and advanced chemical bonding theories will offer a deeper understanding of the complexities of this essential sublevel.
Latest Posts
Latest Posts
-
Is The Atlantic Ocean Warmer Than The Pacific
Mar 15, 2025
-
Que Porcentaje Es 20 De 27
Mar 15, 2025
-
What Is 0 2 As A Percentage
Mar 15, 2025
-
How Many Combinations Of Phone Numbers Are There
Mar 15, 2025
-
Common Multiple Of 3 4 5
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The D Sublevel Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
