How Many Electrons Does A Neutral Atom Of Potassium Contain
Kalali
Mar 23, 2025 · 5 min read
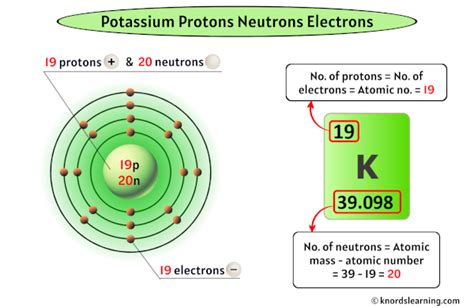
Table of Contents
How Many Electrons Does a Neutral Atom of Potassium Contain? A Deep Dive into Atomic Structure
Potassium, a crucial element for human health and a common ingredient in fertilizers, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is key to grasping its chemical properties and its role in various biological and industrial processes. This article will delve deep into the question: How many electrons does a neutral atom of potassium contain? We'll explore the concept of atomic number, electron shells, valence electrons, and the implications of potassium's electron configuration.
Understanding Atomic Structure: The Foundation of Chemistry
Before answering the central question, let's establish a strong foundation in atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus.
- Neutrons: Neutrally charged particles also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells.
The atomic number of an element is defined as the number of protons in its nucleus. This number is unique to each element and determines its identity. Crucially, in a neutral atom, the number of protons equals the number of electrons, ensuring a balanced overall charge.
Potassium's Place in the Periodic Table: Unveiling its Atomic Number
Potassium (K), located in Group 1 (alkali metals) and Period 4 of the periodic table, holds atomic number 19. This signifies that a neutral potassium atom contains 19 protons in its nucleus. Consequently, a neutral potassium atom also contains 19 electrons.
The Significance of Atomic Number 19
The atomic number 19 is not just a random number; it's a fundamental characteristic that dictates potassium's chemical behavior. It determines the element's position on the periodic table, influencing its reactivity, bonding preferences, and the formation of compounds.
Electron Shells and Subshells: Organizing Electrons
Electrons don't randomly orbit the nucleus; they are arranged in specific energy levels called electron shells. These shells are designated by integers (n = 1, 2, 3, etc.), with shell n=1 being closest to the nucleus and having the lowest energy. Each shell can accommodate a maximum number of electrons, following the formula 2n².
Furthermore, each shell is divided into subshells, denoted by the letters s, p, d, and f. These subshells have slightly different energy levels within a shell. The maximum number of electrons each subshell can hold is:
- s subshell: 2 electrons
- p subshell: 6 electrons
- d subshell: 10 electrons
- f subshell: 14 electrons
Potassium's Electron Configuration: A Detailed Look
Now, let's determine the electron configuration of a potassium atom. With 19 electrons, we fill the shells and subshells in order of increasing energy:
- Shell 1 (n=1): Contains the 1s subshell, which holds a maximum of 2 electrons. Therefore, we fill 1s² (two electrons in the 1s subshell).
- Shell 2 (n=2): Contains the 2s and 2p subshells. The 2s subshell holds 2 electrons (2s²), and the 2p subshell holds 6 electrons (2p⁶). This shell is now full with 8 electrons.
- Shell 3 (n=3): Contains the 3s and 3p subshells. We fill the 3s subshell with 2 electrons (3s²) and the 3p subshell with 6 electrons (3p⁶). This shell also has 8 electrons.
- Shell 4 (n=4): This is where the remaining electron goes. It occupies the 4s subshell, resulting in 4s¹.
Therefore, the complete electron configuration of a neutral potassium atom is 1s²2s²2p⁶3s²3p⁶4s¹. This configuration is crucial in understanding potassium's reactivity.
Valence Electrons: The Key to Reactivity
The outermost shell of an atom, containing the electrons most loosely bound to the nucleus, is known as the valence shell. The electrons in this shell are called valence electrons. These electrons are responsible for the chemical bonding and reactivity of an element.
In potassium, the valence shell is the fourth shell (n=4), containing only one valence electron (4s¹). This single valence electron is easily lost, making potassium highly reactive and prone to forming a +1 cation (K⁺).
The Implications of Potassium's Electron Configuration
The presence of a single valence electron explains many of potassium's properties:
- High Reactivity: Potassium readily loses its valence electron to achieve a stable electron configuration resembling the noble gas argon (1s²2s²2p⁶3s²3p⁶). This loss of an electron leads to the formation of a positively charged ion (K⁺), a characteristic of alkali metals.
- Formation of Ionic Compounds: The strong tendency to lose an electron allows potassium to readily form ionic compounds with nonmetals like chlorine (forming potassium chloride, KCl) and oxygen (forming potassium oxide, K₂O).
- Low Ionization Energy: The energy required to remove the outermost electron (ionization energy) is relatively low for potassium, reflecting its ease of losing its valence electron.
- Metallic Character: Potassium exhibits typical metallic properties like good electrical and thermal conductivity. The freely moving valence electrons contribute to these properties.
Potassium's Role in Biology and Industry
The chemical properties stemming from potassium's electron configuration have significant implications in various fields:
- Biological Importance: Potassium plays a vital role in biological systems. Its ions are crucial for maintaining proper fluid balance, nerve impulse transmission, and muscle contraction. Potassium channels, which regulate the movement of potassium ions across cell membranes, are essential for cell function.
- Industrial Applications: Potassium compounds are widely used in fertilizers to provide essential nutrients to plants. Potassium hydroxide (KOH) is used in various industrial processes, including soap production and as a strong base.
Conclusion: Reinforcing the Core Concept
In conclusion, a neutral atom of potassium contains 19 electrons. This number is directly related to its atomic number (19) and dictates its electron configuration (1s²2s²2p⁶3s²3p⁶4s¹). The presence of a single valence electron in the 4s subshell accounts for potassium's high reactivity, its tendency to form ionic compounds, and its significant role in biological and industrial processes. Understanding the connection between atomic structure, electron configuration, and chemical properties is fundamental to comprehending the behavior of potassium and other elements.
Latest Posts
Latest Posts
-
How Far Is 0 4 Miles To Walk
Jul 12, 2025
-
What Is 20 Percent Of 800 000
Jul 12, 2025
-
Words That Start With Y In Science
Jul 12, 2025
-
Prevent An Expressway Emergency By Merging Without
Jul 12, 2025
-
How Many Grams Of Sugar In A Pound
Jul 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does A Neutral Atom Of Potassium Contain . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.