How Many Electrons Does Carbon Have
Kalali
Mar 12, 2025 · 6 min read
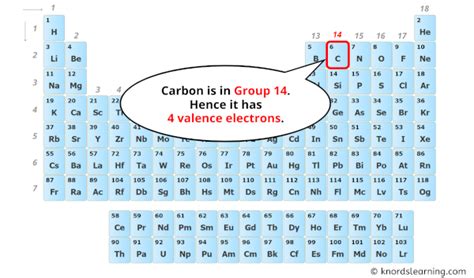
Table of Contents
How Many Electrons Does Carbon Have? A Deep Dive into Atomic Structure
Carbon, the backbone of life and a cornerstone of modern materials science, holds a fascinating position in the periodic table. Understanding its fundamental properties, especially its electron configuration, is crucial to comprehending its remarkable versatility and diverse applications. So, how many electrons does carbon have? The simple answer is six. But the story behind that number is far more complex and revealing. This article delves deep into the world of atomic structure, exploring carbon's electron configuration, its implications for bonding, and its significance in chemistry and beyond.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in carbon, let's establish a foundational understanding of atomic structure. Atoms, the fundamental building blocks of matter, are composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines an element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to an atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. They are significantly lighter than protons and neutrons.
The number of protons and electrons in a neutral atom are always equal, ensuring a balanced charge. It's the arrangement of these electrons that determines an atom's chemical behavior and its ability to form bonds with other atoms.
Carbon's Atomic Number and Electron Configuration
Carbon's atomic number is 6, meaning it has six protons in its nucleus. Consequently, a neutral carbon atom also has six electrons. These electrons are not randomly distributed around the nucleus; they occupy specific energy levels or shells according to the principles of quantum mechanics.
The electron configuration of carbon is 1s²2s²2p². Let's break this down:
- 1s²: The first energy level (n=1) contains the s subshell, which can hold a maximum of two electrons. Both electrons in carbon fill this subshell.
- 2s²: The second energy level (n=2) also has an s subshell, again capable of holding two electrons. These two electrons completely fill the 2s subshell.
- 2p²: The second energy level (n=2) also contains the p subshell, which consists of three orbitals, each capable of holding two electrons. In carbon, only two of these orbitals are occupied, each with one electron. This leaves one orbital empty.
This specific electron configuration is key to understanding carbon's remarkable ability to form four covalent bonds. The two electrons in the 2p subshell are unpaired, allowing carbon to readily share electrons with other atoms to achieve a stable octet (eight electrons in its outermost shell).
Carbon's Bonding Capabilities: The Significance of Four Valence Electrons
The electrons in the outermost shell of an atom are called valence electrons. These are the electrons most involved in chemical bonding. Carbon has four valence electrons – two from the 2s subshell and two from the 2p subshell. This number is crucial to its chemistry.
Carbon's ability to form four covalent bonds stems directly from its four valence electrons. Covalent bonds involve the sharing of electrons between atoms. By sharing its four valence electrons, carbon can achieve a stable electron configuration resembling that of the noble gas neon (with eight valence electrons), a state of maximum stability.
This unique bonding capability allows carbon to form a vast array of molecules, both simple and incredibly complex. This underlies carbon's central role in organic chemistry, the chemistry of carbon-containing compounds.
Carbon's Allotropes: Diverse Forms with Unique Properties
The way carbon atoms bond to each other gives rise to its diverse allotropes – different structural forms of the same element. These allotropes exhibit remarkably different physical and chemical properties, demonstrating the influence of atomic arrangement on macroscopic behavior. Some notable examples include:
- Diamond: A crystalline structure where each carbon atom is bonded tetrahedrally to four other carbon atoms, forming a rigid, three-dimensional network. This accounts for diamond's exceptional hardness and high refractive index.
- Graphite: A layered structure where carbon atoms are bonded in planar hexagonal rings, forming sheets that are weakly bonded to each other. This allows graphite to be soft, slippery, and a good conductor of electricity.
- Fullerenes (e.g., Buckminsterfullerene or "buckyballs"): These are cage-like molecules composed of carbon atoms arranged in spherical, ellipsoidal, or tubular shapes. Fullerenes possess unique electronic and mechanical properties.
- Carbon nanotubes: Cylindrical structures composed of rolled-up sheets of graphene (a single layer of graphite). Their exceptional strength, electrical conductivity, and high aspect ratio make them promising materials for various applications.
Carbon's Importance in Organic Chemistry and Biochemistry
The versatility of carbon's bonding, stemming from its four valence electrons, is the foundation of organic chemistry. The immense diversity of organic molecules—from simple hydrocarbons to complex biomolecules—is a direct consequence of carbon's ability to form strong, stable bonds with itself and other elements such as hydrogen, oxygen, nitrogen, and sulfur.
In biochemistry, carbon plays an indispensable role. The building blocks of life – carbohydrates, lipids, proteins, and nucleic acids – are all fundamentally carbon-based molecules. The intricate structures and functions of these biomolecules are intimately linked to carbon's bonding capabilities. The diversity of life on Earth is a testament to the unique chemical properties of carbon.
Carbon in Materials Science and Nanotechnology
Carbon's unique properties have also led to its widespread use in materials science and nanotechnology. The exceptional strength and electrical conductivity of carbon nanotubes and graphene have spurred significant research and development efforts towards their integration in various technologies. These materials show great promise in fields such as electronics, composites, energy storage, and biomedical engineering.
Isotopes of Carbon: Variations in Neutron Number
While the number of protons and electrons determines an element's chemical identity, the number of neutrons can vary, giving rise to isotopes. Carbon has several isotopes, the most common being:
- Carbon-12 (¹²C): This isotope has six protons and six neutrons and constitutes the vast majority of carbon found in nature.
- Carbon-13 (¹³C): This isotope has six protons and seven neutrons. It's a stable isotope used in various scientific applications, including isotopic tracing.
- Carbon-14 (¹⁴C): This isotope has six protons and eight neutrons. It's radioactive and decays with a known half-life, making it useful for radiocarbon dating.
Conclusion: The Significance of Six Electrons
The seemingly simple answer – carbon has six electrons – belies the immense complexity and significance of this element. Its unique electron configuration, leading to four valence electrons and diverse bonding capabilities, is responsible for carbon's ubiquitous presence and remarkable versatility across chemistry, biology, materials science, and beyond. From the intricate molecules of life to groundbreaking nanomaterials, carbon's six electrons are at the heart of a world of scientific exploration and innovation. Understanding this fundamental aspect of its atomic structure is crucial to appreciating the breadth and depth of carbon's impact on our world.
Latest Posts
Latest Posts
-
What Is 20 Percent Of 100
Mar 12, 2025
-
How Many Feet In 24 Inches
Mar 12, 2025
-
62 Inches Is What In Feet
Mar 12, 2025
-
How Many Quarts Is 2 Gallons
Mar 12, 2025
-
How Many Cups Is A Half A Pint
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Carbon Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
