How Many Electrons Does Chlorine Have
Kalali
Mar 18, 2025 · 5 min read
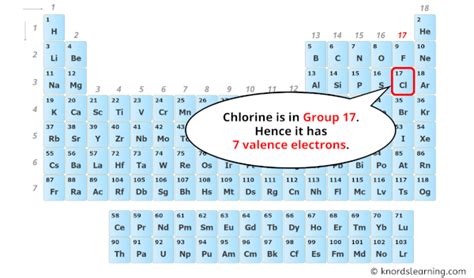
Table of Contents
How Many Electrons Does Chlorine Have? A Deep Dive into Atomic Structure
Chlorine, a vibrant yellow-green gas, plays a crucial role in our daily lives, from purifying drinking water to its presence in essential household products. Understanding its atomic structure, particularly the number of electrons it possesses, is fundamental to comprehending its chemical behavior and reactivity. This article delves into the intricacies of chlorine's electron configuration, exploring its position on the periodic table, its valence electrons, and the implications of its electron count on its chemical properties.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in chlorine, let's establish a foundational understanding of atomic structure. An atom comprises three fundamental subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines an element's atomic number and its identity.
- Neutrons: Neutral particles (no charge) also residing within the nucleus. Neutrons contribute to an atom's mass but not its charge.
- Electrons: Negatively charged particles that orbit the nucleus in specific energy levels or shells. The number of electrons typically equals the number of protons in a neutral atom, ensuring a balanced electrical charge.
Chlorine's Position on the Periodic Table: A Clue to its Electron Count
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. Chlorine (Cl) is located in Group 17 (also known as the halogens) and Period 3. Its atomic number is 17. This atomic number signifies that a neutral chlorine atom possesses 17 protons. Crucially, in a neutral atom, the number of protons and electrons are equal. Therefore, a neutral chlorine atom has 17 electrons.
Electron Shells and Subshells: Chlorine's Electron Configuration
Electrons don't randomly orbit the nucleus; they occupy specific energy levels called shells. These shells have subshells (s, p, d, f) with varying capacities for electrons. The arrangement of electrons in these shells and subshells is known as the electron configuration. For chlorine, the electron configuration is: 1s²2s²2p⁶3s²3p⁵. Let's break this down:
- 1s²: The first shell (n=1) contains the s subshell, which holds a maximum of two electrons. Chlorine has two electrons in this shell.
- 2s²: The second shell (n=2) also has an s subshell with two electrons.
- 2p⁶: The second shell also contains a p subshell, capable of holding up to six electrons. Chlorine fills this subshell completely.
- 3s²: The third shell (n=3) begins with the s subshell, which holds two more electrons.
- 3p⁵: Finally, the p subshell in the third shell contains five electrons. This is where chlorine's reactivity stems from, as it is one electron short of a complete octet (eight electrons) in its outermost shell.
Valence Electrons: The Key to Chlorine's Reactivity
The outermost shell of an atom is crucial in determining its chemical behavior. Electrons in this outermost shell are called valence electrons. For chlorine, the valence shell is the third shell (n=3), and it contains seven electrons (two from the 3s subshell and five from the 3p subshell). This incomplete octet makes chlorine highly reactive. It strongly seeks to gain one electron to achieve a stable, filled octet configuration, making it a highly electronegative element.
Implications of 7 Valence Electrons
The presence of seven valence electrons dictates chlorine's chemical behavior in several ways:
- High Electronegativity: Chlorine has a high electronegativity, meaning it strongly attracts electrons in a chemical bond. This propensity to attract electrons is the driving force behind many of its reactions.
- Oxidation States: Chlorine exhibits various oxidation states, primarily -1 (gaining one electron), +1, +3, +5, and +7. These varying oxidation states reflect its ability to either gain or lose electrons, depending on the reaction conditions and the other elements involved.
- Formation of Ionic and Covalent Bonds: Due to its tendency to gain an electron, chlorine readily forms ionic bonds with metals (by accepting an electron from a metal to become a chloride ion, Cl⁻) and covalent bonds with nonmetals (by sharing electrons).
- Formation of Diatomic Molecules: Chlorine exists naturally as a diatomic molecule (Cl₂), where two chlorine atoms share one pair of electrons to achieve a stable octet configuration. This sharing of electrons results in a covalent bond.
Chlorine's Isotopes and Electron Count
While a neutral chlorine atom always has 17 electrons, it's important to acknowledge the existence of chlorine isotopes. Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. The most common isotopes of chlorine are chlorine-35 (¹⁷Cl) and chlorine-37 (¹⁷Cl). These isotopes have different mass numbers due to the varying neutron counts, but both have 17 electrons in their neutral atomic state. The electron count remains consistent regardless of the isotope.
Beyond the Basics: Chlorine's Role in Chemistry and Everyday Life
The fact that chlorine has 17 electrons, and more specifically, 7 valence electrons, underpins its extensive applications in diverse fields. Here are just a few examples:
- Water Purification: Chlorine is a potent disinfectant, effectively killing harmful bacteria and viruses in drinking water, swimming pools, and wastewater treatment plants. Its reactivity allows it to readily oxidize and inactivate these microorganisms.
- Production of PVC (Polyvinyl Chloride): Chlorine is a key component in the production of PVC, a widely used plastic material in various applications, including pipes, flooring, and clothing.
- Household Cleaning Products: Many household bleaches and disinfectants contain chlorine compounds, leveraging its oxidizing power for effective cleaning and disinfection.
- Pharmaceuticals: Chlorine is involved in the synthesis of various pharmaceuticals and medications.
- Industrial Processes: Chlorine plays a vital role in a variety of industrial processes, including the production of solvents, refrigerants, and other chemicals.
Conclusion: 17 Electrons and a World of Applications
Understanding that chlorine possesses 17 electrons, including its crucial 7 valence electrons, is fundamental to grasping its behavior and properties. This seemingly simple numerical fact provides the key to understanding its remarkable reactivity, its ability to form various bonds, and ultimately, its widespread applications in our daily lives. From purifying our drinking water to enabling the production of essential materials, chlorine's 17 electrons contribute significantly to the world around us. The study of chlorine's atomic structure serves as a powerful illustration of how the fundamental properties of elements dictate their roles in chemistry and in the broader context of our world. Further exploration of the periodic table and its elements reveals similar intricate relationships between electron configuration and chemical behavior.
Latest Posts
Latest Posts
-
Cuanto Es 92 Grados Fahrenheit En Centigrados
Mar 19, 2025
-
How To Get Magnitude Of Force
Mar 19, 2025
-
How Can You Separate Sugar From Water
Mar 19, 2025
-
What Are The Common Multiples Of 9 And 10
Mar 19, 2025
-
What Is 2 Out Of 16 As A Percentage
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Chlorine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
