How Many Electrons Does Oxygen Gain Or Lose
Kalali
Mar 26, 2025 · 5 min read
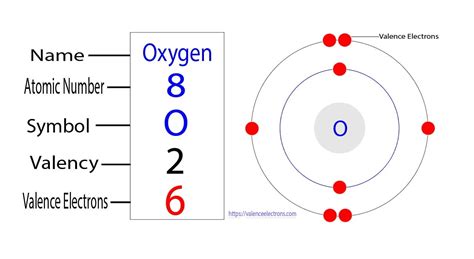
Table of Contents
- How Many Electrons Does Oxygen Gain Or Lose
- Table of Contents
- How Many Electrons Does Oxygen Gain or Lose? Understanding Oxygen's Reactivity
- Oxygen's Electron Configuration: The Key to Reactivity
- Gaining Electrons: The Formation of Oxide Ions
- Examples of Oxide Ion Formation:
- Sharing Electrons: Covalent Bonding in Oxygen Compounds
- Examples of Covalent Bonding with Oxygen:
- The Importance of Oxygen's Electron Behavior
- Conclusion: Oxygen's Versatile Electron Behavior
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons Does Oxygen Gain or Lose? Understanding Oxygen's Reactivity
Oxygen, a vital element for life as we know it, is renowned for its high reactivity. This reactivity stems directly from its electron configuration and its tendency to either gain or share electrons to achieve a stable electron shell. Understanding how many electrons oxygen gains or loses is crucial to comprehending its chemical behavior and its role in various chemical processes.
Oxygen's Electron Configuration: The Key to Reactivity
To understand oxygen's behavior, we need to look at its electron configuration. Oxygen (O) has an atomic number of 8, meaning it possesses 8 protons and 8 electrons in a neutral atom. These electrons are arranged in energy levels or shells: two electrons occupy the first shell (closest to the nucleus), while the remaining six electrons reside in the second shell.
This second shell, however, can accommodate up to eight electrons. This means that oxygen's outermost shell, its valence shell, is only half-filled. Atoms strive for a stable electron configuration, usually resembling that of a noble gas with a full outer shell – the octet rule. For oxygen, achieving this stable octet means either gaining two electrons or sharing two electrons to complete its valence shell.
Gaining Electrons: The Formation of Oxide Ions
The most common way oxygen achieves a stable octet is by gaining two electrons. When this happens, oxygen transforms into an oxide ion (O²⁻). This ion now has 10 electrons (8 original + 2 gained) and 8 protons, resulting in a net charge of -2. This negative charge signifies the gain of electrons.
The formation of oxide ions is a crucial aspect of many chemical reactions. Oxygen's strong electronegativity – its tendency to attract electrons – facilitates this electron gain. The energy released during the formation of oxide ions is significant, contributing to the exothermic nature of many oxygen-related reactions like combustion.
Examples of Oxide Ion Formation:
-
Reaction with metals: When oxygen reacts with many metals, it readily accepts two electrons from the metal atoms, forming a metal oxide. For instance, the reaction between magnesium (Mg) and oxygen (O₂) produces magnesium oxide (MgO):
2Mg(s) + O₂(g) → 2MgO(s)
In this reaction, each magnesium atom loses two electrons to become a Mg²⁺ ion, while each oxygen atom gains two electrons to become an O²⁻ ion. The electrostatic attraction between these oppositely charged ions forms the ionic compound magnesium oxide.
-
Reaction with non-metals: Oxygen can also react with some non-metals to form covalent compounds containing oxide ions. While the electron transfer isn't as complete as in ionic compounds, the oxygen atom still effectively gains a share of electrons, leading to a partial negative charge. Consider the formation of carbon dioxide (CO₂): Each oxygen atom shares two electrons with the carbon atom, effectively achieving a stable octet.
Sharing Electrons: Covalent Bonding in Oxygen Compounds
While gaining electrons is a common pathway for oxygen, it's not the only one. Oxygen can also achieve a stable octet by sharing electrons with other atoms through covalent bonding. This forms molecules rather than ions.
In its diatomic form (O₂), two oxygen atoms share two pairs of electrons (a double bond) to satisfy the octet rule for each atom. Each oxygen atom contributes six valence electrons, and by sharing two pairs, each completes its valence shell with eight electrons. This covalent bonding explains the existence of oxygen gas in its molecular form (O₂).
Examples of Covalent Bonding with Oxygen:
-
Water (H₂O): In water molecules, each oxygen atom forms two single covalent bonds with two hydrogen atoms. The oxygen atom shares one electron with each hydrogen atom, and in turn receives one electron from each hydrogen, effectively completing its octet.
-
Carbon Dioxide (CO₂): As mentioned previously, carbon dioxide exemplifies covalent bonding where each oxygen atom forms a double bond with the carbon atom.
-
Organic Molecules: Oxygen is a crucial component of numerous organic molecules, including alcohols, ethers, ketones, aldehydes, carboxylic acids, and esters. In these molecules, oxygen atoms participate in covalent bonding, often sharing electrons with carbon and hydrogen atoms.
The Importance of Oxygen's Electron Behavior
The ability of oxygen to either gain or share electrons has profound implications across various fields:
-
Combustion: The exothermic reactions involved in combustion are primarily driven by the formation of oxide ions, as oxygen readily accepts electrons from fuels. This process releases a significant amount of energy, making combustion a vital energy source.
-
Respiration: In biological systems, oxygen plays a crucial role in cellular respiration. The process involves the controlled reaction of oxygen with organic molecules (glucose) to produce energy (ATP). This process relies on oxygen's ability to accept electrons, forming water as a byproduct.
-
Oxidation-Reduction Reactions (Redox): Oxygen is a strong oxidizing agent, meaning it readily accepts electrons in redox reactions. Its participation in these reactions drives many chemical and biological processes.
-
Corrosion: The corrosion of metals is often caused by the oxidation of metals by oxygen in the presence of moisture. This leads to the formation of metal oxides, weakening the metal's structure.
Conclusion: Oxygen's Versatile Electron Behavior
Oxygen's chemical behavior is largely dictated by its electron configuration and its strong tendency to achieve a stable octet. It can achieve this stability by either gaining two electrons to form oxide ions (ionic bonding) or sharing electrons through covalent bonding. Understanding this duality is key to comprehending the myriad of chemical and biological processes in which oxygen plays a pivotal role. From combustion to respiration to corrosion, the ability of oxygen to either gain or share electrons underpins its profound influence on our world. Its versatility and importance highlight the fundamental connection between electron configuration and chemical reactivity.
Latest Posts
Latest Posts
-
How Much Water Is 200 Ml
Mar 30, 2025
-
What Is 240 Celsius In Fahrenheit
Mar 30, 2025
-
What Is 40 Off Of 30
Mar 30, 2025
-
What Is 30 Percent Of 12
Mar 30, 2025
-
Cuanto Es 40 Grados Centigrados En Fahrenheit
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Oxygen Gain Or Lose . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
