How Many Grams Are In 4.5 Moles Of Sodium Fluoride
Kalali
Aug 23, 2025 · 5 min read
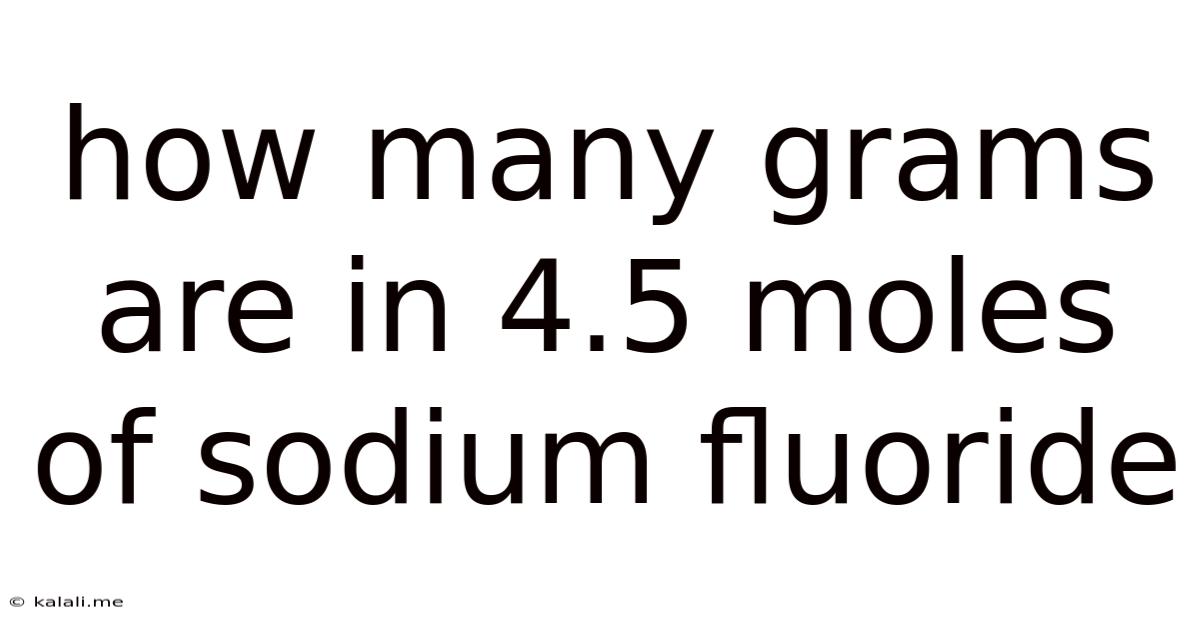
Table of Contents
How Many Grams Are in 4.5 Moles of Sodium Fluoride? A Comprehensive Guide to Mole Conversions
This article will delve into the process of converting moles to grams, specifically focusing on calculating the mass of 4.5 moles of sodium fluoride (NaF). We'll explore the underlying concepts of molar mass, Avogadro's number, and stoichiometry, providing a comprehensive understanding of this crucial chemistry calculation. This guide is ideal for students learning stoichiometry, chemistry enthusiasts, or anyone needing a refresher on mole conversions.
Understanding Moles and Molar Mass
Before we tackle the calculation, let's clarify the fundamental concepts. A mole (mol) is a fundamental unit in chemistry representing a specific number of particles, namely Avogadro's number (approximately 6.022 x 10²³). This means one mole of any substance contains 6.022 x 10²³ atoms, molecules, ions, or formula units.
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It's essentially the atomic weight (or the sum of atomic weights for compounds) expressed in grams. For example, the molar mass of carbon (C) is approximately 12.01 g/mol because one mole of carbon atoms weighs 12.01 grams.
Calculating the Molar Mass of Sodium Fluoride (NaF)
To determine the molar mass of NaF, we need the atomic masses of sodium (Na) and fluorine (F) from the periodic table. The atomic mass of sodium is approximately 22.99 g/mol, and the atomic mass of fluorine is approximately 19.00 g/mol.
Therefore, the molar mass of NaF is:
22.99 g/mol (Na) + 19.00 g/mol (F) = 41.99 g/mol (NaF)
Converting Moles to Grams: The Formula
The conversion between moles and grams is straightforward:
Grams = Moles x Molar Mass
This formula allows us to calculate the mass of any substance given the number of moles and its molar mass. Conversely, we can calculate moles given the mass and molar mass.
Calculating the Mass of 4.5 Moles of Sodium Fluoride
Now, let's apply this formula to our problem: calculating the mass of 4.5 moles of NaF. We already know the molar mass of NaF is 41.99 g/mol.
Grams of NaF = 4.5 mol x 41.99 g/mol
Grams of NaF ≈ 189 grams
Therefore, 4.5 moles of sodium fluoride has a mass of approximately 189 grams.
Significance and Applications
This simple calculation has significant implications in various chemical applications. Accurate mole-to-gram conversions are crucial for:
-
Stoichiometric Calculations: In chemical reactions, stoichiometry uses mole ratios to determine the amounts of reactants and products. Accurate mass calculations are essential for controlling reaction yields and avoiding waste. For example, if you are reacting NaF with another substance, knowing the precise mass of NaF you're using is critical for determining the amount of product formed.
-
Solution Preparation: Preparing solutions with specific concentrations often requires precise mass measurements. The concentration of a solution is typically expressed in terms of molarity (moles of solute per liter of solution). To prepare a solution of a certain molarity, you'll need to calculate the mass of the solute (NaF in this case) required.
-
Analytical Chemistry: Many analytical techniques, such as titration and gravimetric analysis, involve measuring the mass of substances to determine their concentration or purity. A precise understanding of mole-to-gram conversions is essential for accurate analysis.
-
Industrial Processes: Chemical industries rely on precise mass calculations for efficient and safe production. This is critical in quality control and ensuring the consistency of products.
Beyond the Basics: Addressing Potential Errors and Refinements
While the calculation above provides a good approximation, several factors can influence the precision of the result:
-
Significant Figures: The accuracy of the final answer depends on the number of significant figures used in the calculations. Using more significant figures for atomic masses will yield a more precise result. In our calculation, we used four significant figures. Depending on the context, you might need to adjust this for greater accuracy.
-
Isotopic Abundance: Atomic masses listed on the periodic table are weighted averages that account for the different isotopes of an element. The actual mass of a sample of NaF may slightly vary due to the specific isotopic composition. This variation is usually negligible for most practical purposes.
-
Experimental Error: In a real-world laboratory setting, measurements of mass and volume will always have some degree of experimental error. This error can propagate through the calculations and affect the final result. Proper laboratory techniques and calibrated instruments minimize this error.
Further Exploration: Related Concepts and Calculations
Understanding mole-to-gram conversions is a cornerstone of many other important chemical calculations, including:
-
Percent Composition: Calculating the percentage of each element in a compound.
-
Empirical and Molecular Formulas: Determining the simplest and actual formula of a compound using mass data.
-
Limiting Reactants: Identifying the reactant that limits the amount of product formed in a chemical reaction.
-
Theoretical and Percent Yield: Comparing the actual yield of a reaction to the theoretical maximum yield.
Conclusion
Calculating the mass of 4.5 moles of sodium fluoride demonstrates the practical application of fundamental chemistry principles. The conversion between moles and grams is a crucial skill in various fields, from academic research to industrial applications. Mastering this conversion allows for accurate calculations, precise experimental design, and a deeper understanding of chemical reactions and processes. By understanding the underlying concepts and acknowledging potential sources of error, you can confidently perform these conversions and apply them to a wider range of chemical problems. Remember to always utilize the most accurate atomic masses available and consider significant figures to ensure the precision of your calculations. This detailed explanation provides a solid foundation for further exploration of stoichiometry and other advanced chemical concepts.
Latest Posts
Latest Posts
-
How Do You Write 0 7 As A Fraction
Aug 23, 2025
-
How Fast Can A 90cc Atv Go
Aug 23, 2025
-
What Is The Best Example Of A Narrative
Aug 23, 2025
-
Can A Decimal Be A Natural Number
Aug 23, 2025
-
How Far Will A 380 Bullet Travel
Aug 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Grams Are In 4.5 Moles Of Sodium Fluoride . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.