How Many Neutrons Are In Boron
Kalali
Apr 07, 2025 · 5 min read
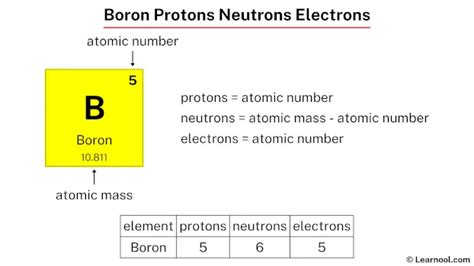
Table of Contents
How Many Neutrons are in Boron? A Deep Dive into Isotopes and Nuclear Physics
Boron, a metalloid element with the symbol B and atomic number 5, presents a fascinating study in nuclear physics due to its isotopic composition. Unlike many elements with one or two dominant isotopes, boron boasts two naturally occurring isotopes: boron-10 and boron-11. This variation significantly impacts the number of neutrons present in a boron atom, making it a compelling topic for exploration. This article delves deep into the world of boron isotopes, neutron count, nuclear properties, and the applications stemming from this unique elemental characteristic.
Understanding Isotopes: The Key to Neutron Count Variation
Before we pinpoint the neutron count in boron, let's solidify our understanding of isotopes. Isotopes are atoms of the same element that possess the same number of protons (defining the element) but differ in the number of neutrons. This neutron variation alters the atom's mass number (protons + neutrons) but not its chemical properties.
The Atomic Nucleus: A Tale of Protons and Neutrons
The nucleus of an atom, the central core, houses both protons and neutrons. Protons carry a positive charge, while neutrons are electrically neutral. The number of protons determines an element's atomic number and its place on the periodic table. Neutrons, on the other hand, contribute to the atom's mass but don't influence its chemical behavior.
Boron's Isotopic Duo: Boron-10 and Boron-11
Boron exhibits two stable isotopes:
-
Boron-10 (¹⁰B): This isotope accounts for approximately 19.9% of naturally occurring boron. Its nucleus contains 5 protons and 5 neutrons (10 - 5 = 5 neutrons).
-
Boron-11 (¹¹B): The more abundant isotope, comprising about 80.1% of natural boron. It has 5 protons and 6 neutrons (11 - 5 = 6 neutrons).
Calculating Neutron Count: A Simple Formula
Determining the number of neutrons in an isotope is straightforward:
Number of Neutrons = Mass Number - Atomic Number
Where:
- Mass Number: The total number of protons and neutrons in the nucleus (represented as a superscript before the element's symbol).
- Atomic Number: The number of protons (unique to each element and found on the periodic table).
Applying this formula to boron's isotopes:
- ¹⁰B: 10 (mass number) - 5 (atomic number) = 5 neutrons
- ¹¹B: 11 (mass number) - 5 (atomic number) = 6 neutrons
Therefore, a boron atom can contain either 5 or 6 neutrons, depending on the isotope.
The Significance of Isotopic Abundance in Boron's Properties
The relative abundance of boron-10 and boron-11 significantly impacts the overall properties of naturally occurring boron. This varying neutron count influences several aspects:
-
Atomic Weight: The weighted average of the masses of the isotopes, considering their relative abundance, determines boron's atomic weight, approximately 10.81 amu (atomic mass units).
-
Nuclear Reactions: The difference in neutron numbers affects how boron isotopes react in nuclear processes. Boron-10, for instance, is a strong neutron absorber, making it crucial in nuclear reactors as a control rod material and in radiation shielding. This high neutron absorption capacity is directly related to its specific nuclear structure and neutron count.
-
Applications in Materials Science: The neutron count influences the properties of boron-containing materials, affecting their strength, reactivity, and other characteristics, leading to diverse applications in various industries. The isotopic composition is crucial when tailoring materials for specific applications.
Boron's Applications: From Nuclear Reactors to Semiconductors
The unique isotopic nature of boron, particularly its neutron absorption properties, makes it invaluable in numerous fields:
1. Nuclear Technology:
-
Neutron Absorbers: Boron-10's high neutron capture cross-section makes it vital in controlling nuclear chain reactions in reactors. Boron carbide (B₄C) is frequently employed in control rods to regulate the rate of fission.
-
Radiation Shielding: Boron's neutron absorption capacity also plays a significant role in shielding against neutron radiation, protecting personnel and equipment from harmful exposure.
2. Materials Science:
-
Boron Fibers: High-strength boron fibers, reinforced with carbon or other materials, find application in advanced composite materials for aerospace and other high-performance applications. The precise isotopic composition can be tailored to optimize the material's properties.
-
Boron-doped Silicon: Boron is a key dopant in semiconductor manufacturing. Adding boron to silicon creates p-type semiconductors, essential components of transistors and integrated circuits. The precise level of boron doping, influenced by the isotopic composition, is critical for controlling the electrical properties of the semiconductor.
3. Other Applications:
- Detergents: Boron compounds are used in detergents and bleaches.
- Glass and Ceramics: Boron is a component in many types of glass and ceramic materials.
- Medicine: Boron compounds are employed in boron neutron capture therapy (BNCT), a targeted cancer treatment.
Advanced Isotopic Analysis Techniques
Precise determination of the isotopic composition of boron requires sophisticated techniques. These methods are crucial for quality control in applications where the exact neutron count and isotopic ratio are critical, such as in nuclear technology and semiconductor manufacturing:
-
Mass Spectrometry: This technique separates ions based on their mass-to-charge ratio. By analyzing the relative abundance of ¹⁰B and ¹¹B ions, one can accurately determine the isotopic composition.
-
Neutron Activation Analysis (NAA): This technique utilizes neutron bombardment to induce radioactivity in the sample. By analyzing the resulting radioactive emissions, the isotopic abundances can be inferred.
-
Inductively Coupled Plasma Mass Spectrometry (ICP-MS): This highly sensitive method combines inductively coupled plasma with mass spectrometry, allowing precise measurement of boron isotopes in various matrices.
These advanced techniques ensure precise control over the isotopic composition, crucial for applications requiring specific nuclear properties or tailored material characteristics.
Further Exploration: Unstable Boron Isotopes and Nuclear Research
While ¹⁰B and ¹¹B are the only stable isotopes of boron, several unstable (radioactive) isotopes exist. These isotopes have shorter half-lives and undergo radioactive decay, emitting various particles and energy. The study of these unstable isotopes offers valuable insights into nuclear processes, decay mechanisms, and nuclear structure. Research into these isotopes contributes to our understanding of nuclear physics and the fundamental forces governing the universe.
Conclusion: The Significance of Neutron Count in Boron's Multifaceted Role
The number of neutrons in a boron atom, determined by its isotopic identity (either 5 or 6), directly influences its properties and applications. The varying neutron counts of ¹⁰B and ¹¹B affect atomic weight, nuclear reactivity, and material properties. This makes boron a fascinating element for study, with its unique isotopic composition contributing significantly to its versatility and importance in diverse fields, from nuclear technology and materials science to medicine and industrial applications. The continued investigation of boron's isotopic properties promises further advancements in various technological sectors and fundamental scientific understanding.
Latest Posts
Latest Posts
-
How Many Feet Are 30 Inches
Apr 07, 2025
-
How Many Inches Is 44 Centimeters
Apr 07, 2025
-
What Is 0 25 As A Percent
Apr 07, 2025
-
1 Meter 92 Cm In Feet
Apr 07, 2025
-
How Many Minutes Is 14 Hours
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Boron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.